Table of Contents
Cranmer
Lawyer with over 25 years experience in some of the world’s biggest law firms. I divide my time between the UK and NZ.
Covid is a controversial and emotive topic. People hold strong views often based on bitter or difficult personal experiences. In the midst of the pandemic, there were no perfect solutions but experts and officials worked under intense pressure to provide their advice to government. A broad array of recommendations covering numerous issues needed to be synthesized into coherent pandemic policies for approval by Cabinet.
My intention is not to second-guess the science or the decisions made by experts but to examine what recommendations were made by the relevant technical committees and then compare those with how the government decided to act and how those actions were presented to the public by the Prime Minister, the former Director-General of Health, Dr Bloomfield and others.
I will refer to the minutes of relevant Cabinet and committee meetings which are publicly available. Those documents demonstrate that key recommendations made by the government’s technical experts were not implemented in relation to:
- increasing the dose interval between the first and second dose from three weeks to at least eight weeks for the under 30s in order to reduce the risk of myocarditis; and
- permitting the under 18s to have only one dose for the purpose of workplace mandates.
In addition, there is clear evidence in the official documents that it was agreed that references to increasing dosing intervals as a method of potentially providing some protection against myocarditis should be removed from public communications.
Finally, there are unanswered questions about why CV TAG spent months planning to recommend the vaccination of ‘high risk’ 12 to 15-year-olds only to abruptly change its mind without explanation three days before the relevant Cabinet meeting.
Given the length of this article, I have broken it into two. I will publish the second half of the article tomorrow which will deal with the question of permitting the under 18s to have a single dose for the purpose of workplace mandates.
Delta arrives
Tuesday, 17 August 2021 was another unremarkable day until Prime Minister Ardern and Dr Bloomfield announced to the nation at 6 pm that a case of Delta had been detected in the community and that New Zealand would therefore enter a level 4 lockdown from 11:59 pm that evening. It signalled a step-change in the country’s Covid response which saw weeks of daily press conferences, commentary and frenzied activity concerning the spread of Delta, the acceleration of the vaccination programme and the logistical implications of the lockdown.
The positive sample which triggered the lockdown had been provided by a member of the public at 1:45 pm on 16 August. Coincidently Cabinet was meeting at the same time to decide whether to use the Pfizer vaccine for children aged 12 to 15. On the face of the official papers, the decision looked straightforward.
Medsafe had already granted provisional approval for use of the Pfizer vaccine in children aged 12 to 15 on 1 June and Dr Bloomfield had then requested advice from the Covid-19 Vaccine Technical Advisory Group (CV TAG) as part of the Decision to Use process.
The Cabinet paper for 16 August, prepared by New Zealand’s Covid Minister, Chris Hipkins noted that, “subsequent advice from CV TAG in August, supported the inclusion of all children aged 12 to 15 years in the Immunisation Programme”. The paper continued, “based on the advice received from the Director-General of Health, I recommend that Cabinet agree to proceed with using the Pfizer vaccine for children aged 12 to 15 years”.
At a press conference at 1 pm on 19 August, Prime Minister Ardern announced the decision to the country in the following manner:
As you know, it’s imperative that we get as many people as possible vaccinated. When we make a decision on who is eligible, though, our No. 1 priority is the medical advice of our experts. You will remember that in June, our regulator, Medsafe, granted provisional approval for the Pfizer vaccine to be given to 12 to 15 year-olds in New Zealand. Similar decisions have been made by other regulators in Europe, the US, Canada and Japan. The advice was then considered by an additional group of experts, who also supported an extension of eligibility to young people. On that basis, Cabinet has agreed to make the Pfizer vaccine available for 12 to 15-year olds. This is not a decision we have taken lightly.
Many of us are parents ourselves and take this duty of making decisions about other people’s children extremely seriously, but it is safe, and it’s the right thing to do. So 12 to 15 year olds can become eligible and book, along with everyone else that we are opening up to from the 1st of September.
The Prime Minister stressed that the first priority was the medical advice of our experts but how accurate was her claim?
Extension of vaccination to children aged 12 to 15 years
To put CV TAG into context, it is an advisory group comprised of medical and scientific experts which provides advice and recommendations specifically in relation to vaccinations. It is chaired by Dr Ian Town, the Chief Science Advisor at the Ministry of Health and included Associate Professor Helen Petousis-Harris from the University of Auckland. CV TAG considers a range of recommendations formulated by ministry officials and then provides advice to a Ministry of Health steering group formerly chaired by Dr Bloomfield which is responsible for senior-level decision-making for the Covid-19 Immunisation Programme. The steering committee and the Director-General then provide advice to the Vaccine Ministers and Cabinet.
CV TAG had considered whether to recommend extending vaccinations to the 12 to 15-year-old age group from June 2021. In their meeting of 22 June, CV TAG recommended deferring vaccination of this age group for several reasons, noting that “there is a potential safety signal for myocarditis in people under 30 years who receive mRNA vaccines (e.g., Pfizer/BioNtech and Moderna), which requires ongoing consideration”.
On 20 July CV TAG again advised that vaccination of the 12 to 15-year-old age group should be deferred. CV TAG was however preparing draft recommendations in anticipation of giving the green light to priority ‘high risk’ groups at a later date. The minutes recorded:
- “Draft recommendations for potential priority groups among children were shared with the group to inform the Decision to Use for 12 to 15-year-olds.”
- “Priority groups overseas have included children who are about to undertake long-term immunosuppression, immunocompromised, in long term residential care, requiring transplants or who have neurological disabilities or gastrointestinal (multi-system medically vulnerable) conditions. Risk factors for Covid-19 such as obesity, respiratory disease and ethnicity should also be taken into account.”
On 3 August CV TAG made the decision to recommend extending vaccination to high priority sub-groups within the 12 to 15 year old age cohort as they had been planning to do for several months. The minutes noted:
- “Earlier advice has been that a broader decision on vaccinating 12 to 15-year-olds should be deferred.”
- “An exception should be made for priority groups of 12 to 15-year-olds that are at a higher risk from Covid-19 due to prior comorbidities, as outlined in the draft memo, which CV TAG supported.”
The next day Dr Town, as Chair of CV TAG, provided a memo to Joanne Gibbs, the Director of National Operations of the COVID Vaccine Immunisation Programme confirming the group’s recommendations.
The memo, titled ‘Priority groups for vaccination among 12- to 15-year-olds: COVID-19 Vaccine Technical Advisory Group (CV TAG) recommendations on the use of the Pfizer vaccine’ was copied to Drs Bloomfield and McElnay. It stated:

Of note, the memo concluded:

On 2 August, the Australian Technical Advisory Group on Immunisation (ATAGI) made a very similar decision as CV TAG made on 3 August to approve vaccination of children aged 12 to 15 who were in high risk sub-groups.
It, therefore, appears unusual that on 13 August – three days before the Cabinet meeting – Dr Town sent Dr Bloomfield a brief one page memo titled ‘Extending the age groups who can receive Covid-19 vaccine’ which appeared to override the recommendations made in CV TAG’s memo nine days earlier. Instead of vaccinating ‘high risk’ 12 to 15 year-olds CV TAG was now recommending that all children in that age group should be vaccinated.
The short memo simply stated:

The memo appears almost deliberately ambiguous in its brevity. It does not explain why CV TAG “convened by email” nor what the reason was for CV TAG’s abrupt change of mind after months of planning.
Its lack of detail suggests that this was the result of a hasty political decision that was being rubber-stamped by the advisory group.
The effect of CV TAG’s 12 August decision was that it jumped ahead of their UK and Australian counterparts in the approval of vaccination of all 12 to 15-year-olds. It wasn’t until 27 August, that ATAGI finally approved the extension of vaccination to that age group with a recommended dose interval of three to six weeks.
At that point in time, the UK’s Joint Committee on Vaccination and Immunisation (JCVI) was still only recommending vaccinating high risk sub-groups in the 12 to 15-year-old cohort.
The JCVI explained their cautious approach in a press release on 3 September as follows:
“A precautionary approach was agreed given the very low risk of serious disease in those aged 12 to 15 years without an underlying health condition that puts them at increased risk. Given this very low risk, considerations on the potential harms and benefits of vaccination are very finely balanced.”
“The margin of benefit, based primarily on a health perspective, is considered too small to support advice on a universal programme of vaccination of otherwise healthy 12 to 15-year-old children at this time. As longer-term data on potential adverse reactions accrue, greater certainty may allow for a reconsideration of the benefits and harms. Such data may not be available for several months.”
The caution shown by the relevant UK and Australian technical committees explains the lingering doubt recorded in the minutes of CV TAG’s 21 September meeting about their decision to extend the rollout to all 12 to 15-year-olds:

One consequence of Cabinet’s seemingly premature decision to approve the vaccination of all 12 to 15-year-old children was that it got ahead of the consideration and implementation of extending dose intervals and/or the implementation of a single dose protocol, which in each case was recommended by CV TAG in order to reduce the risk of myocarditis in younger people. Those issues are set out in more detail below.
The myocarditis risk
Medsafe, New Zealand’s medicines regulator first started to receive advice on the myocarditis risk on 24 May 2021 when it was advised of “two cases of potential myocarditis associated with the Comirnaty (Pfizer/BioNTech) vaccination”. The Independent Safety Monitoring Board (CV ISMB), chaired by John Tait, convened on 27 May to consider this and other safety signals.
During CV TAG’s 20 July meeting, the group also considered the myocarditis risk. The next day, the Chair of the group, Dr Town, sent Dr Bloomfield a memo headed ‘Myocarditis following vaccination: COVID-19 Vaccine Technical Advisory Group (CV TAG) recommendations on the use of the Pfizer vaccine’.
The memo notes that “Emerging data from countries such as the United States of America (USA) and Israel, indicate that there is a risk of myocarditis and/or pericarditis following Pfizer and Moderna mRNA COVID-19 vaccination. The risk appears to be higher following the second dose, in males and in younger age groups.”
The first recommendation of CV TAG set out in paragraph 24(a) of their 21 July memo was that the interval between the first and second dose should be extended to at least eight weeks for people aged 16 to 29:

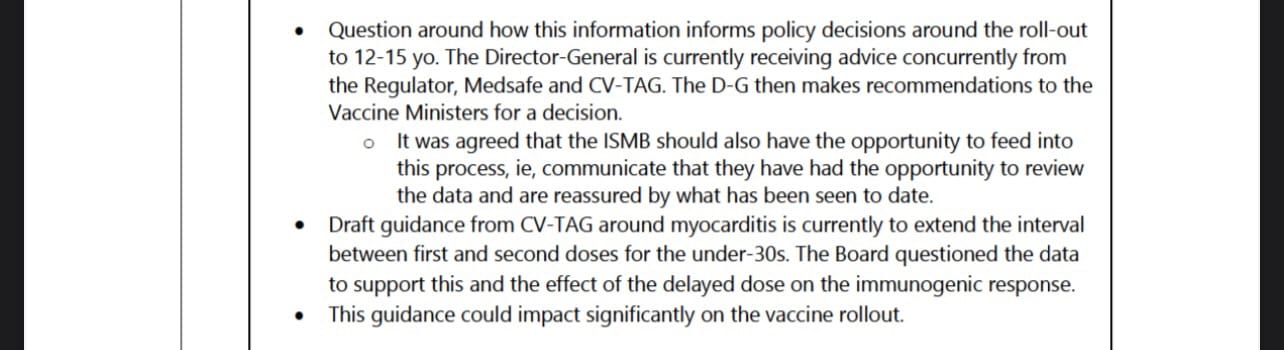
CV ISMB also met on 21 July and the minutes of their meeting, set out below, noted that CV TAG’s recommendation to extend the dose interval could have a significant impact on the vaccine rollout.
That observation made by CV ISMB appears to highlight the tensions, described in more detail below, that played out over the next few months within government between increasing the dose interval to mitigate the risk of myocarditis on the one hand and accelerating the vaccine rollout on the other.
Despite slightly differing views between CV TAG and CV ISMB, on 12 August Dr Bloomfield publicly announced that the interval between the first and second dose of vaccine had been extended from three weeks to six weeks. Without giving any specifics, Bloomfield noted that, “a small number of well-designed studies show that an extended duration between doses of the Pfizer vaccine gives at least an equally robust immune response, with no additional safety concerns”.
The press release continues: “The larger interval is also consistent with the advice from the COVID Technical Advisory Group (CVTAG) for an extended interval between doses.”
Another NZ government press release on the same date is identical to the Ministry of Health communication but in relation to the sentence quoted above, it includes a reference to myocarditis and pericarditis:

The inconsistency in the two press releases is explained by the minutes of CV TAG’s 17 August meeting which somewhat concerningly records that a request had been made that references to increasing dosing intervals as a method of potentially providing some protection against myocarditis should be removed from public communications:

Who made this request, and on what basis was it in the public interest to remove information which would inform the public of the reason why a sensible action could be taken to mitigate against a known risk? It appears that a communications strategy took precedent over providing accurate medical advice to the public which was a necessary element of informed consent.
The request also suggests that there was a reluctance within government to fully articulate the myocarditis risk to young people, provide them with advice, for instance, on resting after vaccination as was given in Singapore, and give consideration to offering the adenovirus vector vaccine to young males.
On 6 October another seemingly inexplicable decision was announced. The government reversed its position on dose intervals by announcing that it was going back to a three-week recommendation for everyone eligible for vaccination. It left journalists in the parliamentary press conference that day utterly perplexed as to what was happening. Please listen to Minister Hipkins and Dr McElnay answer questions from journalists on this point. McElnay states:
… we certainly have sought further advice from our technical advisory group on this particular issue because a number of health professionals have said to us at this moment in time when um we really want to get as many people as possible fully vaccinated, ‘can people be vaccinated before the six weeks?’ And the technical advisory group has come back and said ‘yes, there never was any um, ah, safety concerns with um vaccinating at the three week’ and so from a practical, pragmatic perspective at this moment in time if you’ve had your first dose, and the only thing that’s stopping you from getting your second dose is waiting for a six weeks mark, the advice is that that can be done sooner …
Really, Dr McElnay?
Because that statement is completely at odds with the formal recommendations set out in the CV TAG memo titled ‘Myocarditis following vaccination: COVID-19 Vaccine Technical Advisory Group (CV TAG) recommendations on the use of the Pfizer vaccine’. McElnay received a copy of that memo on 21 July.
Although McElnay and the government attempted to link their decision to reduce the dose intervals to CV TAG, in truth it was a political decision. CV TAG, as an advisory group, was one of many competing voices that was vying for influence over the government’s Covid policy at that time. Differing opinions existed within other advisory groups despite the fact that those groups may not have been providing formal recommendations on this particular issue. For example, the minutes of CV ISMB set out above illustrates a difference of emphasis with regard to dose intervals and vaccine rollout.
The media, including a variety of medically qualified commentators, were examples of other such voices that had varying degrees of influence within government. In some cases, those medical commentators were offering opinions on issues that were outside their areas of expertise and some held views on vaccination protocols that differed from those held by some of the government’s technical experts on CV TAG.
There were effectively two competing schools of thought that existed inside and outside government on this issue. Proponents of maintaining New Zealand’s elimination policy, which included some experts within government and the majority of media commentators, advocated for vaccination that followed Pfizer’s FDA-approved, recommended dosing schedule of 2 doses with a three week interval.
Others, including some of the government’s technical experts, favoured the more flexible policy approach of the UK, Canada and Australia which each considered and recommended longer dosing intervals that differed from the supplier’s recommended schedule on the basis that emerging studies showed that the longer interval of eight weeks or more elicited stronger vaccine efficacy and reduced the myocarditis risk in younger people. CV TAG’s recommendation of an eight week or longer dose interval for the under 30s, as set out in its memo of 21 July, suggests that it was also in this camp.
The longer dose recommendation would not have prevented high risk individuals, or those that simply wished to be vaccinated sooner, from taking two doses at any time after a period of three weeks had passed between doses assuming of course that they had been fully informed of the myocarditis risk, stratified by age and sex.
Politically, however, the government bounced about on the issue. It began by following Pfizer’s recommended dosing schedule of a three week interval. It then ignored CV TAG’s recommendation of at least eight weeks for the under 30s that was made in mid-July, instead recommending a six week interval to the public in mid-August before finally reverting back to a three week interval in early October.
With the benefit of hindsight, CV TAG’s recommendation made in July 2021 seems to have been the right call from an efficacy and safety perspective. The position that it recommended is also consistent with the international consensus.
For example, Australia’s ATAGI now recommends that “all adolescents aged 12-15 years complete a primary vaccine course of 2 doses of COVID-19 vaccine, 8 weeks apart.”
The UK is even more cautious than Australia. Its current health advice is as follows:
“The NHS is offering COVID-19 vaccine to children and young people aged 12 to 17 years. Young people at greater risk of serious illness if they catch COVID-19 will need 2 doses of vaccine, 8 weeks apart. All other young people will be offered 2 doses of vaccine 12 weeks apart.”
New Zealand’s policy reversal, however, obviously had potentially significant implications for people under 30, including for those who were subject to mandates that required them to be vaccinated with two doses not more than six weeks apart despite a CV TAG recommendation that they be permitted at least an eight-week interval due to their increased risk of myocarditis.
It also affected children aged 12 to 15 who had only recently been approved for vaccination at a time when the recommended dose interval had been extended to six weeks and who were also within the age group that was at an increased risk of myocarditis from the shorter dose interval.
Tomorrow Part 2 of this article will consider CV TAG’s recommendation to permit the under 18s to have a single dose for the purposes of the workplace mandates.