Table of Contents
Maryanne Demasi
Maryanne Demasi, 2023 Brownstone Fellow, is an investigative medical reporter with a PhD in rheumatology, who writes for online media and top tiered medical journals. For over a decade, she produced TV documentaries for the Australian Broadcasting Corporation (ABC) and has worked as a speechwriter and political advisor for the South Australian Science Minister.
Of all the antiviral drugs for Covid-19, Pfizer’s Paxlovid has been the most successful. Not for its safety and efficacy, but for its ability to earn the company billions in profits despite being largely ineffective for most people.
In November 2021, before any data had emerged, the Biden Administration committed to purchasing 10 million treatment courses of Paxlovid worth $5.3 billion, pending authorisation by the US drug regulator.
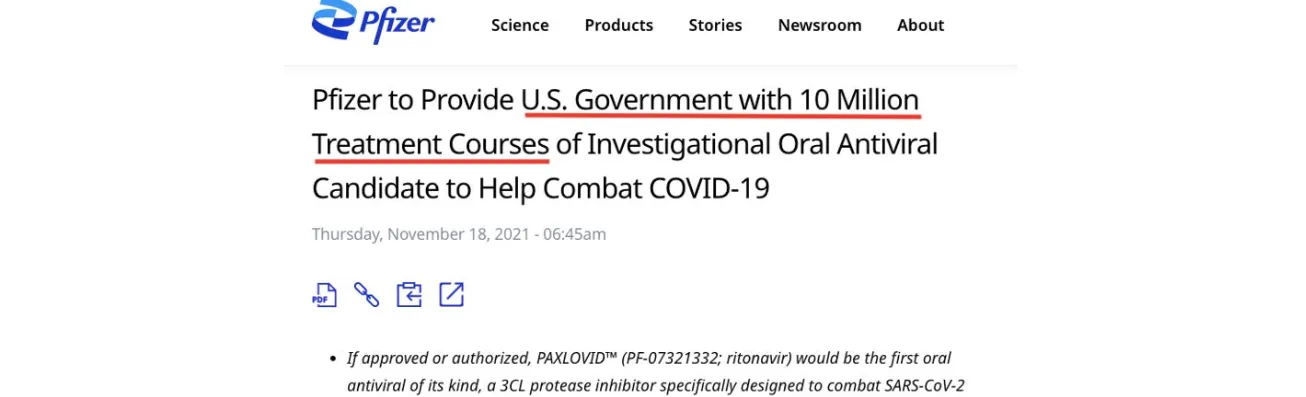
One month later, Paxlovid was granted emergency use authorisation (EUA) by the FDA for use in adult and paediatric populations, 12 years or older.
The authorisation was based on early trial data showing the drug could reduce hospitalisations or death (89 per cent relative risk reduction, six per cent absolute risk reduction) in high-risk patients who were unvaccinated and had no prior exposure to Covid-19.
But the problem was, most Americans by that time (Dec 2021) had already been vaccinated against Covid-19 or had prior exposure to the virus, making the trial results irrelevant to the majority of people.
Pfizer had to prove its drug could benefit a broader market.
The manufacturer commenced the EPIC-SR trial, investigating the use of Paxlovid in unvaccinated people and vaccinated people with at least one risk factor for Covid-19 [clinicaltrials.gov].
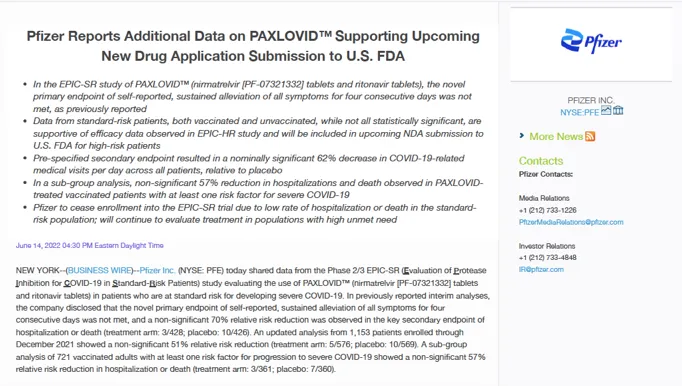
By July 2022, however, Pfizer stopped enrolling participants “due to a very low rate of hospitalization or death observed in the standard-risk patient population.”
In a press release, the company announced that Paxlovid failed to impact its “novel primary endpoint of self-reported, sustained alleviation of all symptoms for four consecutive days.”
In other words, Paxlovid – a combination of nirmatrelvir and ritonavir – made no significant difference in alleviating symptoms of Covid-19 compared to placebo among non-hospitalised patients.
Pfizer stated that it was difficult to find benefit in a population that was already at a low rate of hospitalisation or death from Covid-19.
One year later, in August 2023, Pfizer quietly published the unfavourable findings on clinicaltrials.gov, without any fanfare or media attention. In fact, the media continued to promote the benefits of Paxlovid to the wider public.
The New York Times, for example, ran multiple stories during the pandemic about the ‘Power of Paxlovid,’ encouraging more people to take the drug and criticised its under-use.

Simultaneously, Pfizer stoked public fear by overinflating the risk of Covid-19, paving the way for doctors to prescribe drugs like Paxlovid to manage the disease. Sometimes, the claims were misleading.
Pfizer, for example, tweeted that three out of four American adults were at “high risk” for severe Covid-19, but then cited a study in the advertisement that did not support the claim – so far, the misleading tweet has been viewed 11.6 million times.

“This is ridiculous,” tweeted Walid Gellad, Professor of Medicine at the University of Pittsburgh, “I don’t know how it is legal… three out of four adults are not at high risk of severe Covid.”
That didn’t stop FDA commissioner Robert Califf from taking to social media to promote the benefits of Paxlovid.
He tweeted the drug could reduce the risk of developing ‘long covid’ based on weak evidence, and admitted to ‘cheerleading’ the use of Paxlovid because he felt overall “the evidence was strong.”
Califf copped criticism for his lack of impartiality as the head of the regulator, but justified his actions in a “public health emergency.”
Regulatory affairs expert Jessica Adams said it was a poor excuse.
“Something is really wrong with public health ‘leadership’ if it thinks that every norm can be thrown out the window in an emergency,” said Adams. “The FDA has learned nothing during the pandemic and is setting terrible precedents for future emergencies.”
By 2023, reports of people experiencing ‘rebound’ symptoms after using Paxlovid were increasing. Authorities could no longer claim it was ‘rare.’
High-profile officials such as former CDC director Rochelle Walensky, former NIAID director Tony Fauci, President Joe Biden and First Lady Jill Biden all had reported a rebound of Covid symptoms after completing a course of Paxlovid.

Califf dismissed concerns about rebound, saying it was all just a “distraction,” but a study published in JAMA Network showed that symptomatic rebound in people with mild to moderate Covid-19 was as high as 25 per cent after taking Paxlovid.
In May 2023, the FDA granted Paxlovid full approval for managing mild to moderate Covid-19 infections in adults at high risk of developing severe disease (including vaccinated adults, despite no data showing benefit in this population).
Last week, Paxlovid was back in the spotlight after the EPIC-SR trial was finally published in the New England Journal of Medicine, almost two years after Pfizer announced the futility of the study in July 2022.
Regardless of all the positive media coverage and promotion of Paxlovid by public health officials and government advisors, the evidence is clear.
Paxlovid, which now costs $1,400 for a five-day course, has only shown benefit in a very rare population – that is, unvaccinated people who’ve never encountered the virus and are at high risk of serious Covid-19.
Republished from the author’s Substack.