Table of Contents
OPINION
THE PROBLEM WITH SECRETLY PRODUCED LEGISLATION.
We can now demonstrate how the over-riding Covid-19 goal for officials, from August 2020 onwards, was high vaccine coverage. The power and resources directed through New Zealand’s most powerful ministries to support the ‘largest immunisation programme undertaken in New Zealand’ was further enabled by organisational structures with privileges that enabled them to sequester information – knowledge – from the public. The making of secondary legislation, the Ministerial Orders, and the justification for the production of the mandates was always secret.
What is also coming to light, are the ways policy-making systems were decoupled from traditional legal, scientific and public health principles.
The result was a whole of government juggernaut, revolving around a single agenda, that was at such scale and pace it was impossible for officials and government employees to reject. That was so opaque it was almost impossible for the public to challenge. It could be observed in the secrecy of decision-making, and the capture of scientific information, and with it, the media and the judiciary.
This was not a public health operation which subscribed to traditional public health principles. It was top-down and authoritarian. It was techno-optimistic, with the vaccine purchase strategy led by Ministry of Business, Innovation and Employment, the Ministry of Foreign Affairs and Trade and the Ministry of Health. We were solving the pandemic problem by subjecting every adult to the same treatment.
What minor official would contradict this power? The human and financial resources dedicated to vaccine uptake vastly exceeded resources which might contradict scientific or promotional claims or challenge the global vaccination agenda.
It was ethically bankrupt because it prevented us from judging risk for healthy people. The State pressure on healthy pregnant women to receive the novel bioengineered genetic therapy was particularly immoral. New Zealand’s friendly propaganda urging pregnant women to get vaccinated sharply contrasted with the harmful vaccine profile known by Pfizer and governments in early 2021. The vaccine could harm the mother, neonate and newborn baby (through breastmilk).
New Zealand information, including the Immunisation Handbook, swept all pregnant women into an elevated risk category (despite referencing reviews that highlighted comorbidity risk). Although risk for pregnant women was stratified to multimorbidity and socio-economic status government officials failed to contrast absolute risk from vaccination alongside background risk by age and health status. In addition, officials relied on pharmacovigilance reports but largely ignored injury risk reported in the peer reviewed literature.
Risk should have been judged by family doctors who best knew the patient was at risk because they had higher rates of previous preterm birth, stillbirth, neonatal death and pre-existing medical conditions. Instead, mandates, distancing and propaganda directed families to vaccination, distorting the principle of informed consent.
Doctors traditionally do not give new drugs to pregnant women, because of uncertainty around harm. An older person who is harmed from a drug may only suffer for years, but neonatal harm can impair life quality for decades. The novel bioengineered genetic therapy should have been accorded the same caution. Mothers were assured that the spike protein wouldn’t leave the injection site. But biodistribution studies were never done on where synthetic spike protein end up in the body. Because the trials had not finished, Pfizer explicitly stated:
‘currently insufficient data to make conclusions about the safety of the vaccine in subpopulations such as children less than 16 years of age, pregnant and lactating individuals, and immunocompromised individuals’.
Developing fetuses are exquisitely sensitive to toxic insults, even at parts per billion or trillion. Adverse effects may be unknown or risk downplayed. The lesson of the hormone disrupting drug Diethylstilbestrol (Thalidomide), including the difficulty of victims in seeking redress is well known. State funding for researching environmental harms is minimal, and there are no scientists with expertise and secure core funding who might speak up.
The laws developed to support the campaign made it almost impossible for the public to contradict through judicial review, because the laws revolved around chasing infection, rather than protection of health and preventing hospitalisation and disease in the most vulnerable.
The public unease at the rollout plan, first understood as the ‘sequencing framework’, contrasts sharply with government messaging that hard-line tactics were justified. Most unease surrounded the mandatory deployment of the experimental medication, a novel mRNA, a novel bioengineered genetic therapy (coded BNT162b2) that, contrary to older, traditional vaccines, instructs the body to reproduce an S-protein, a spike protein. The scientific literature has recognised the harmful potential of the spike protein for over a decade.
The departure away from ordinary democratic processes that promote trust [and] the decoupling from principles has been extraordinary over the Covid-19 era. Possibly the strangest decoupling was the act by the Attorney-General, list MP David Parker, to remove obligations that ministers had to abide by the Health Act when making Orders.
Important protections & principles jettisoned.
Parker achieved this via the secret, overnight, May 13, 2021 Covid-19 Public Health Response Bill. The Covid-19 Public Health Response Act (the Covid-19 Act) ensured that Orders produced under section 11 were not required to adhere to traditional obligations and principles required under the Health Act 1956. This was deliberately done by deliberately removing the authority of the Health Act relating to Orders (and therefore mandates).
13 (1) A section 11 order may not be held invalid just because–
(a) it is, or authorises any act or omission that is, inconsistent with the Health Act 1956 or any other enactment relevant to the subject matter of the order; or
(b) it confers any discretion on, or allows any matter to be determined, approved or exempted by any person
This scythed off a requirement to consider individual health, particularly the impact on young people and pregnant women. First, the obligation to improve, promote and protect public health would not be required as a mandatory consideration. Judicial review of actions taken using the power of the Orders simply had to fulfill the purpose of the overnight Covid-19 Act. Secondly, the highly relevant overarching principles (see Part 3A, Subpart 1), required to be considered by officials when managing an infectious disease, were not required to be considered either.
That these two rather obvious considerations could be jettisoned, so lightly and so effortlessly, by overnight legislation, is my pick for the single most important policy and legislative failure.
Instead, the key Covid-19 legislation gave power to ministers and officials to take action that would prevent, and limit the risk of, the outbreak or spread of Covid-19, and action that would avoid, mitigate or remedy the actual or potential adverse effects of the Covid-19 outbreak.
‘Adverse effect’ was never defined to include adverse effects from interventions as time progressed, particularly for people who were not at risk of hospitalisation and death. There was no requirement to associate an outbreak with severe death and/or disease, because this wasn’t required by the World Health Organisation. The infectious agent didn’t have to send people to hospital, for these laws to be made. The laws revolved around officials dealing with a Covid-19 outbreak.
Relatedly, no distinction was made in the legislation between Sars-CoV-2 outbreaks of infectivity, and the disease, Covid-19, for which, in March 2020, most people were not at risk of.
Astonishingly, in drug development, safety always is the primary consideration, then efficacy. No matter how effective a drug is, it has to be safe. We can’t always depend on the drug producer, which is why it is important to methodologically review the literature as an emergency event progresses, including from different study designs, and triangulate the evidence. It’s not enough to rely on claims of the manufacturer or pull together reports that have no methodological basis and invite accusations of cherry-picking. But there was no stopping point, when injury and death from the novel bioengineered genetic therapy would signal that the injection campaign should cease. No such considerations were in place in the legislation – particularly, in Chris Hipkins’ vaccinations legislation.
This inevitably meant that applicants for judicial review could never ask for review based on the fact that actions were not protecting health, that the response did not have to be proportionate and consider the individual, which were obligations in the Health Act. The overriding legislation never discerned that people were differently at risk to respiratory viruses, just like they always had been. Parker and Hipkins put in place blunt instruments.
But the legislation gave officials and ministers extraordinary powers.
MINISTERIAL PRIVILEGE & SECRECY
Government policy and law was constructed swiftly and secretly through meetings of the Executive Council. As the highest formal instrument of government, the Executive Council sets in play Orders in Council, which are then sent to the Parliamentary Counsel Office for drafting. Theoretically Cabinet must approve all matters for consideration by the Executive Council.
But every bit of this pathway was carried out in secret. What is rather eye-opening, as the Cabinet Manual (2017, and the newer 2023 edition) notes, is that the Executive Council only require only two ministers in attendance to constitute a quorum, plus the presiding officer. Prior to the Covid-19 era, orders which would impact the entire population had never been produced.
The ministers’ belief that each new order was justified, never had to be publicly justified. No supporting information that would provide a background, such as a regulatory impact statement, had to be supplied. It’s evident that impartial, procedural reviews were never undertaken, which would include reviews of the scientific literature that might reveal how the novel bioengineered genetic therapy impacted the human body. Scientists globally, were working hard to draw attention to all aspects of risk and treatment throughout the pandemic.
But because of the way our legislation was constructed, research which might triangulate Pfizers’ claims of efficacy and safety was out of scope. Market authorisation had been based on company data, and New Zealand’s overriding legislation did not require broad-based reviews that shed light on the published scientific knowledge concerning the safety and efficacy of the novel bioengineered genetic therapy.
This dark space – the Cabinet discussions we can’t see, the company data we can’t see – enabled the weaponization of Ministerial Orders (which produced the mandates) to an extraordinary degree. The public cannot even access the dates orders were instructed to be produced, such is the extent of secrecy.
At the same time the corporations who entered into vaccine supply contracts have secured a privilege the public could not dream of. From early discussions with vaccine lobby groups, to contract negotiation and signing, to the Pfizer/BioNTech safety and efficacy data, and then the unfortunate post-marketing results. Even the financial losses of stale vaccines, remain hidden. It’s all kept secret.
The secret post-marketing report.
By traditional standards, Pfizer’s February 2021 post-marketing report should have triggered a safety review. Post-marketing reports had to be supplied to Medsafe within five working days of being produced. It’s been difficult to establish when the New Zealand version of the post-marketing-adverse-event-up-to-February 28, 2021 information drop might have arrived, with New Zealand officials claiming that the FDA document was produced for a specific legal purpose. Officials seem to be skirting around the issue.
The inconvenient post-marketing information may have arrived at about the same time the March 10, 2021 Rollout Plan was released. The plan lodged an expectation that civil society would be injected. The government intended for the entire population to be injected with strange new technology by signing an advance purchase agreement for 8.5 million doses.
Over the next year, the production of orders were always consistent with this expectation, and the timeline.
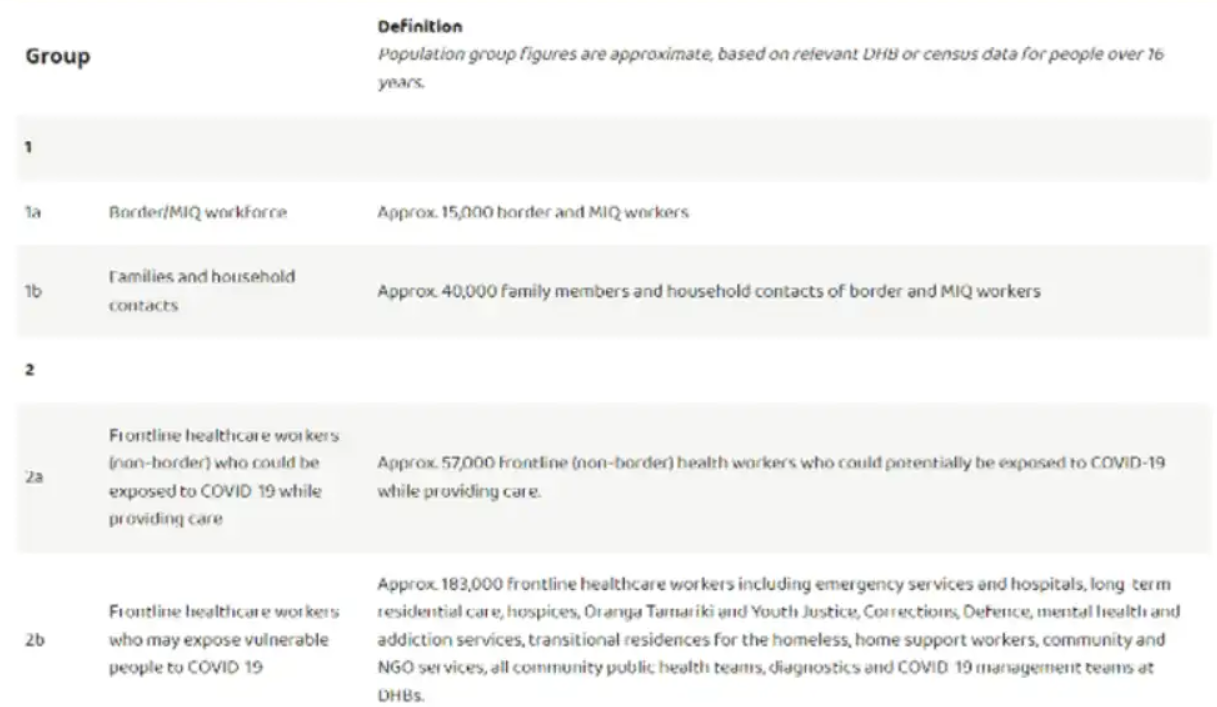
Officials must not shy from briefing ministers on controversial matters. The Department of Prime Minister and Cabinet (DPMC), should have been briefed of the newly understood and alarming harm profile revealed in the February 28 2021 post-marketing report, and this information should have been held with the DPMC.
But they refused to answer my request for memos or advice directly relating to the Pfizer/BioNTech February 28, 2021 post-marketing report. Noting that, of course, the mandates came from decisions made by the Executive Council. This information was relevant to decisions to widen mandates.
My March OIA request to the Ministry of Health sought to identify discussions relating to the post-marketing data, as I recognised the ministry have been reluctant to publish this data.
Strangely, the Ministry of Health flicked part [a] of the request to Te Whatu Ora, who wasn’t invented at the time of the request.
Please supply urgently, between 27 February 2021 and 9 March 2021 the following information:
[a] All discussions via WhatsApp, Signal, Messenger, private email, Parliamentary or
political party email by officials, including Ministers and the Director General of Health
discussing Pfizer, BioNTech, the post-marketing report, FDA, deaths and adverse events.
This was rejected by Te Whatu Ora. Apparently, my request for ‘reports, releases, memos and advice’ between 27 February 2021 and 9 March 2021 would ‘require substantial collation due to the hundreds of employees and potentially thousands of Messages’.
The second part of this request (H2023021708) concerned access to
[b]All reports, releases, memos, advice discussing and/or reviewing the implications of, contents, evidence in the post-marketing report supplied as a requirement in the Gazette conditions, and taking all steps to reasonably consider the harm profile to the population who were not at risk of hospitalisation or death from Covid-19 (required by Health Act, Part3A, s.92, Overarching Principles). This includes the risk to pregnant women, young people and children.
The Ministry of Health rejected my request on the following grounds:
- section 9(2)(b)(ii) where its release would likely unreasonably prejudice the commercial position of the person who supplied the information.
- section 18(f) as the information requested cannot be made available without substantial collation or research.
- section 9(2)(a) to protect the privacy of natural persons
Then I considered that an understanding of the department and dates might be reasonable. But the response to that OIA request (HR2023022884) confirmed that the public are not permitted to [know] who was responsible for managing this up to February 28, 2021 information drop; this was refused as it might lead to improper pressure or harassment. Nor are we permitted know on what dates the post-marketing information arrived. I did not specifically say adverse events to February, 28, yet I suspect that James and the Ministry of Health understood very well that post-marketing information concerned the same information as that that was in the FDA’s Cumulative Analysis of Post-authorization Adverse Event Reports.
We cannot know the information was produced [and] the dates Medsafe released it. We cannot risk the reputation of the manufacturer and, strangely, official staff privacy needs to be protected.
The dates when ministers decided to produce the next mandates order are secret too.
The extent to which we are not permitted to understand the information used by responsible ministers to justify the orders, was highlighted when I attempted to understand the dates when orders were first instructed to be made. By understanding the dates, I could perhaps establish what information was received by now Prime Minister, but minister at-the-time of the Covid-19 Response, Chris Hipkins to inform him that [there was] sufficient evidence to justify the increasing mandates.
I made Official Information Act requests to the Parliamentary Counsel Office, the DPMC and the Ministry of Health. I wanted, simply, to understand the date instructions were made. To firstly, (A) make editorial changes to all reprints of the Covid-19 Public Health Response (Vaccinations) Order 2021 from 14 July 2021 onwards, and secondly (B) Commence drafting of: Covid-19 Response (Vaccinations) Legislation Bill 2021.
I didn’t ask for the contents, which of course would be secret. Just the dates. But once again the DPMC flicked my request to the Ministry of Health, even though of course the order decisions come out of Executive Council meetings.
Therefore when I expressed surprise that the information would have been sent through ministers and/or Cabinet officials, the DPMC Ministerial Coordinator acknowledged that:
The request was transferred, following consultation with the Ministry of Health, as it is more closely connected to their work and functions. The transfer does not mean the information was not sent through Ministers or Cabinet officials. A response will be provided by the Ministry of Health which will provide details of this work.
Even though the Ministry of Health administers both the Covid-19 Public Health Response (Vaccinations) Order 2021 and the Covid-19 Response (Vaccinations) Legislation Act 2021, this information would have been held by the DPMC. Remember: the Executive Council, the council which comprises all Ministers of the Crown, has a principal function of advising the Governor-General to make orders in Council.
The instructions are then passed to the Parliamentary Counsel Office (PCO), who are responsible for drafting most of the documents for the Executive Counsel. I was aware that the PCO were not subject to the Official Information Act but I wondered if the date of each instruction would be hidden by confidentiality rules. The PCO responded with:
This isn’t something we can help you with, however. Drafting instructions, and related communications between the instructor and PCO, are confidential and subject to legal professional privilege (see section 131 of the Legislation Act 2019).
The Ministry of Health then responded to confirm that the dates were subject to legal and professional privilege. Chief Legal Advisor Phil Knipe noted that he did consider “the countervailing public interest in releasing these drafting instructions and consider that it does not outweigh the need to withhold at this time”.
The question begs, on what basis? Knipe directed me to this page, which contains no information in relation to the orders requiring affected persons to be injected, and then that the public carry CVCs, Covid-19 Vaccination Certificates. Information specifically concerning mandate orders was not on this page.
Such is the extent of secrecy. A law that required everybody to submit to a medical treatment should be underpinned by impeccable reasoning. The decision to make that law, will be understood at that time, and then later, in courts of law, to be reasonable and fair-minded.
But we are not permitted to be privy to that information.
By the time the rollouts and accompanying laws were being made, there was substantial intelligence available that the emergency event had been overstated, that and most of the population were not at risk from Covid-19. The chosen medical treatment, a novel genetic therapy that instructed the body to reproduce a spike protein, was only one small element of the novel coronavirus. The technology was never designed, nor efficacy based on prevention of transmission.
But the Director-General, Minister for Health & Minister for Covid-19 – should have known.
Yet by mid-2021 Minister for Covid-19, and now Prime Minister Chris Hipkins, should have been shown the devastating post-marketing study that revealed unexpectedly high death and injury rates, including for pregnant women and neonates.
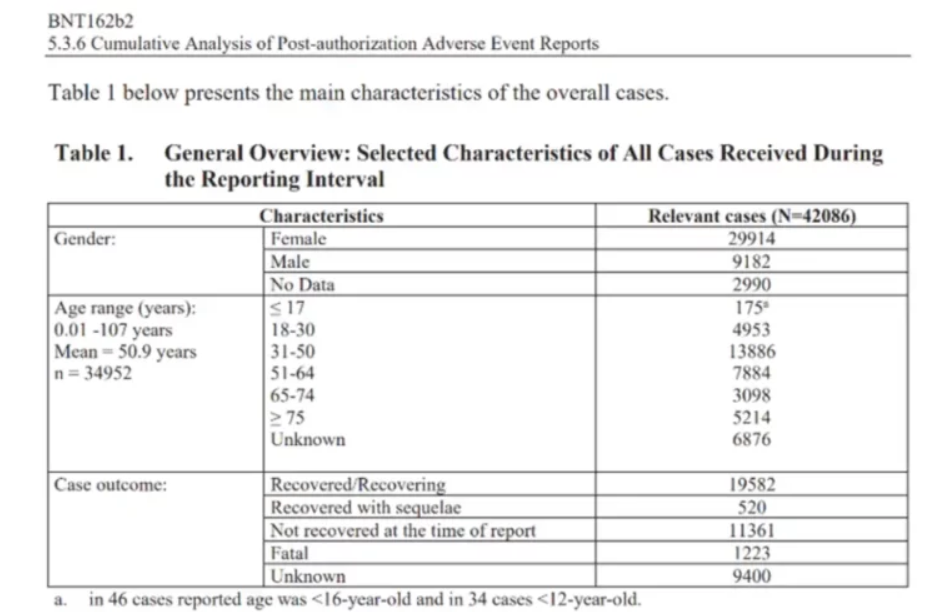
Governments should have had early (pre-publication) access to Pfizer’s six-month trial data that revealed a higher death rate in the vaccine group than the placebo group, long before the data was formally published for public viewing.
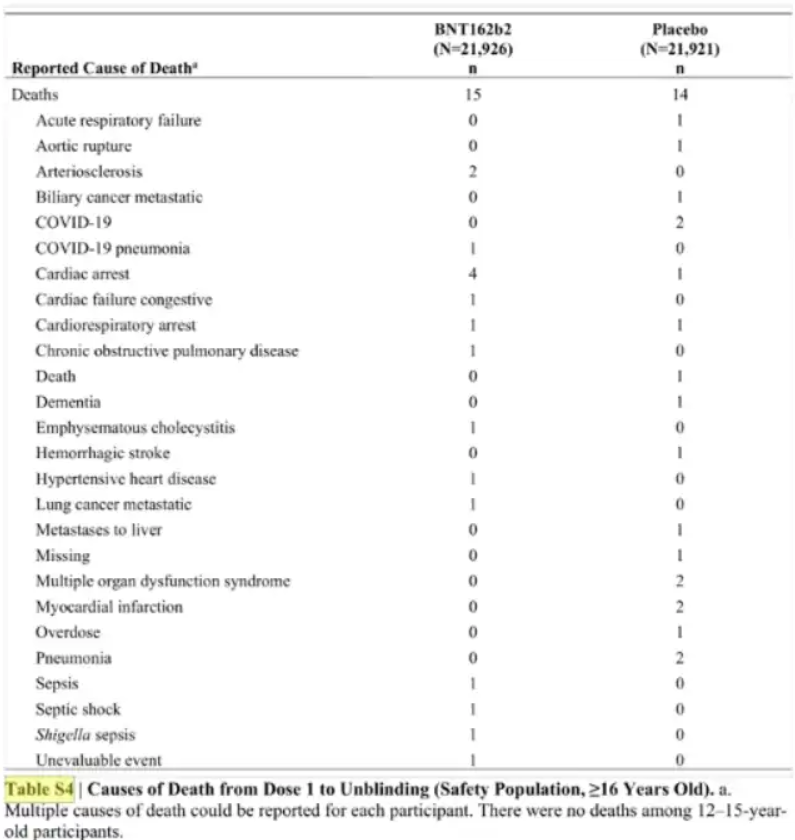
Why should Hipkins have been directly shown this information? Because he was putting in place law demanding the public were injected. Laws demanding that pregnant women, even though officials recognised that trials in pregnancy had not been completed and that pregnant women should clearly be informed of risks, should be vaccinated to work. Officials had an obligation to show him contradictory information.
As I have demonstrated, I’ve been unsuccessful in attempting to any reviews or analysis of the implication for official decision-makers that might have been disrupted after New Zealand received our version of the FDA post-marketing report. I now consider it was buried.
There were no rolling reviews of the changing science relating to safety and efficacy: I asked Pharmac and spent enough time chasing the Ministry of Health to establish if there were methodological reviews of the changing scientific information. They’re not there.
Civil society would expect, logically, that the massive post-marketing information dump from Pfizer to the FDA would be in the form of a single report, and that New Zealand would have also received a single report. We can’t know because the barriers to us knowing are mighty.
The courts generally agree that limits such as mandates must serve a sufficiently important objective or purpose that warrants overriding a protected right or freedom, and that the means chosen to achieve the objective must be proportionate. With regards to the latter, the means chosen must be rationally connected to the objective, there must be minimal impairment and the effect must be proportionate. The benefits achieved must not be outweighed by the significance of the limitation of the right.
This, the public would think, hinges on information. The provision of science.
But what remains obscured is the purpose and scope that gives ministers and officials their powers; which then sets the priorities, in this case, on chasing after a respiratory infection of a quickly mutating virus using a novel bioengineered genetic therapy.
Yes, officials must not be biased, and must consider controversial information. Indeed,
An authority may unlawfully abdicate its statutory function by refusing or failing to act. A public body must not renounce its decision-making responsibility, nor preclude itself from inquiring into matters relevant to its inquiry. [Constitutional and Administrative Law in New Zealand, 4th Ed., P.A Joseph, 23.2.3, p.972]
The non-disclosures, the obfuscation of information and the barriers to the production of negative information that could contradict whole of government messaging do not portend well for the next emergency event. Because what we have seen over Covid-19 is an absolute failure of the Government to honestly report on the epidemiological evidence, who was most at risk of Covid-19, and why the experimental mRNA technology was unlikely to support the health of the most at-risk.
While the power to release orders was amplified, the secrecy remained. To quote sociologist Samantha Crompvoets: “Power and knowledge, or rather the concealment of knowledge – secrecy – are two significant predictors of misconduct that can be seen in a number of institutional contexts.”
But I consider that by this time officials were already numb, blandly avoiding inconvenient facts, including the absence of data on pregnant women, the likelihood it might not work on multimorbid populations, the absence of genotoxicity and carcinogenicity studies and the reality that safety and efficacy was based on limited symptoms one week after injection.
With such the prevailing organisational structure (including legislation, policies and formal and informal power relations) in place, when the government did receive bad news there was no obligation to disclose it. Complete obfuscation around when this highly controversial post-marketing report/s were supplied to the secret people who were responsible for receiving the secret data, remains.
For several years the New Zealand public have squabbled over the legitimacy of mandates and whether the right to refuse medical treatment could be justified. In the courts, on social media, in families, churches and workplaces.
It’s more than that: it’s the capacity, entrenched in legislation and in protocols, for elite groups to act secretly, with little oversight and no accountability. This has been enhanced by wizardly green curtain of scientific complexity, which often could be simplified by the following of Health Act principles, and public health principles, but which weren’t. Crompvoets informs us further:
The more elite, secretive and cloistered a group is, the higher the chance of deviation, and the concealment of that deviation. This is exacerbated when the group or subgroups hold specialist skills that are not well understood by those outside of the organisation, and are revered or despised.
It’s not about the culture, it’s about the rules that let the players play the game. And over Covid-19, while knowledge was changing on a weekly basis, the rules were structured by the players to supercharge the production of laws outside public processes, while maintaining secrecy.
What lockdowns, what surveillance and what injections will be imposed on the New Zealand public in the next emergency?








![[The Good Oil] March 2026 Political Poll](https://images.unsplash.com/photo-1540910419892-4a36d2c3266c?crop=entropy&cs=tinysrgb&fit=max&fm=webp&ixid=M3wxMTc3M3wwfDF8c2VhcmNofDk1fHx2b3Rpbmd8ZW58MHx8fHwxNzIyMzkxNTI4fDA&ixlib=rb-4.0.3&q=80&w=1304)
