Table of Contents
Gary Moller
Gary Moller is a Health Practitioner who is focused on addressing the root causes of ill health or poor performance by making use of a key forensic tool – Hair Tissue Mineral Analysis – and administering healthy, natural and sustainable therapies.
If we get it wrong, the TPA’s Orwellian Regulator has some big sticks to strike us with:
A two million dollar fine. Five years in prison and confiscation and destruction of our assets.
If we’re going to continue to make health claims and dispense natural health products, we’d better watch what we say or do, because these penalties are kill shots.
The Act threatens free speech. Here is but one example:
“Pt 8. Cl 249 (also refer 187 (2)) This clause appears to make it illegal to criticise a product publicly on the threat of conviction and/or a fine not exceeding $200,000. This is completely contrary to having the right to free speech, and to ‘call out’ the failings of a product or government to meet the requisite thresholds in the event of mass enforcement of a product.”
Quoted from the submission about the TPA by Sandra Goudie (27/1/2023)
Retired Mayor and Member of Parliament. Her submission, which has guided this article, is reproduced in full at the bottom of this article. Thank you, Sandra!
There’s growing awareness and concern over the regulation of nutritional supplements and the impact it may have on free speech regarding their health benefits. The New Zealand government introduced the Therapeutic Products Act earlier this year, aiming to provide a regulatory framework for therapeutic products. While the intention behind the Act is to guarantee the safety and efficacy of such products, there are concerns that it may stifle free speech. This article delves into the potential issues surrounding the Therapeutic Products Act, focussing on the little-understood Part 6, which deals with prohibited conduct, a topic I am only now getting my head around.
The Rush to Legislation
A remarkable feature of the Therapeutic Products Act was its lightning-fast approval process. Despite an overwhelming 13,000 submissions from the public, the select committee managed to draft a report in a mere three days. This breakneck pace has left many questioning the depth of legislative procedure and the true value of public input. It leads us to ponder:
Whose interests are our Members of Parliament truly representing? The people of New Zealand, or perhaps others? Big Pharma immediately springs to mind.
Part 6: A Cause for Concern
Part 6 of the Therapeutic Products Act, titled “Other Prohibited Conduct,” has become a focal point for those concerned about free speech regarding nutritional supplements. Two sections, in particular, have been a cause for concern: Section 190 regarding tampering with products and Section 193 addressing misrepresentation.
Section 190: To Tamper With

Section 190 deals with the act of tampering with therapeutic products. While this may seem like a reasonable provision to protect consumers from dangerous alterations to products, the concern arises when one considers how broadly this section could be interpreted. Could public comments or discussions about the efficacy of a product be considered tampering, even if there is no physical alteration of the product itself?
Section 193: Misrepresentation
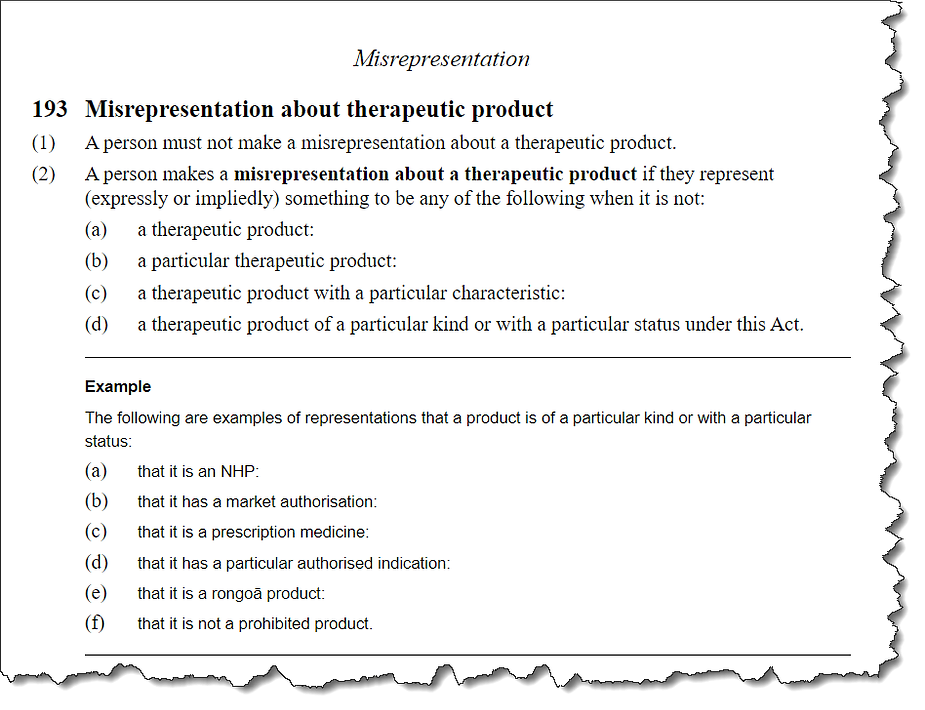
Section 193 focuses on misrepresentation, which includes any false or misleading statements about therapeutic products. While it’s essential to prevent deceptive marketing practices, the worry here is that this section might inadvertently hinder individuals from sharing their genuine experiences and opinions about these products. Will honest testimonials and discussions about the benefits of nutritional supplements be considered misrepresentation under this provision?
A Threat to Free Speech
The Therapeutic Products Act, with its broad language and rapid legislative process, has raised legitimate concerns about its impact on free speech regarding nutritional supplements. While the Act aims to guarantee the safety and efficacy of therapeutic products, it must strike a balance with individuals’ right to express their opinions and share their experiences.
While some may argue against our concerns, suggesting that the Regulator will wield their punitive authority fairly and judiciously, one must consider who’d willingly step forward to be the proverbial guinea pig in testing this law and, in the process, establishing a precedent in Case Law. I say to those proponents: “Be my guest.” But consider the potential consequences — shelling out hundreds of thousands in legal fees, enduring years of exclusion from your business, and facing the peril of losing not only your livelihood but also your reputation and even your freedom!” Health professionals, like myself, and NZ-based importers, manufacturers, and wholesalers will likely choose to exit the health sector, rather than risk being prosecuted.
Conclusion
The New Zealand Therapeutic Products Act, though well-intentioned, has ignited a debate about its potential to stifle free speech regarding the health benefits of nutritional supplements. As the Act is implemented and interpreted, it’s crucial for individuals, advocates, and lawmakers to remain vigilant in safeguarding the fundamental right to express opinions and share experiences without fear of legal repercussions.
Balancing safety and efficacy with free speech will be a challenging but necessary task for New Zealand’s evolving regulatory landscape. Looking at what they have given us with the TPA, I think our lawmakers could do a lot better.
Note: NZ First has committed to overturning these laws, provided they secure enough seats to do so. They have a history of following through on their promises, as demonstrated with past iterations of this Act. Therefore, they represent our sole chance for relief. We are throwing our full support behind NZ First, and we hope you’ll join us in doing so.
Give your Party vote to NZ First and your Electorate vote to the person who you feel best represents you and has a good chance of winning their seat. If not, then give that vote to NZ First.











