Table of Contents
Sven Román
brownstone.org
Sven Román is a child and adolescent psychiatrist and since 2015 a consultant psychiatrist working in child and adolescent psychiatry throughout Sweden.
A recent review of phase III randomized trials of Covid-19 mRNA vaccines showed an increased risk of hospitalization for the vaccinated compared to controls.1 Data from the Swedish Medical Products Agency (LV) confirms that Covid-19 vaccines have resulted in a distinct increase in reported side effects, including suspected serious side effects (SSSEs) and deaths. Despite this, the consistent message from the Swedish Public Health Agency (FoHM) is that benefit outweighs risk. Does the evidence support this assertion?
With the aim of determining the relative risk of experiencing a SSSE including death, versus becoming hospitalized or dying with Covid-19, we analyzed publicly available data from LV, FoHM, the Swedish National Board of Health and Welfare (SoS) and Statistics Sweden (SCB). Assuming that five per cent of all SSSEs are reported in Sweden, our analysis shows that Covid-19 vaccination is likely to benefit one single group only – men aged over 90 years. For all other groups, the incidence of SSSEs exceeded the risk of hospitalization and death with COVID-19.
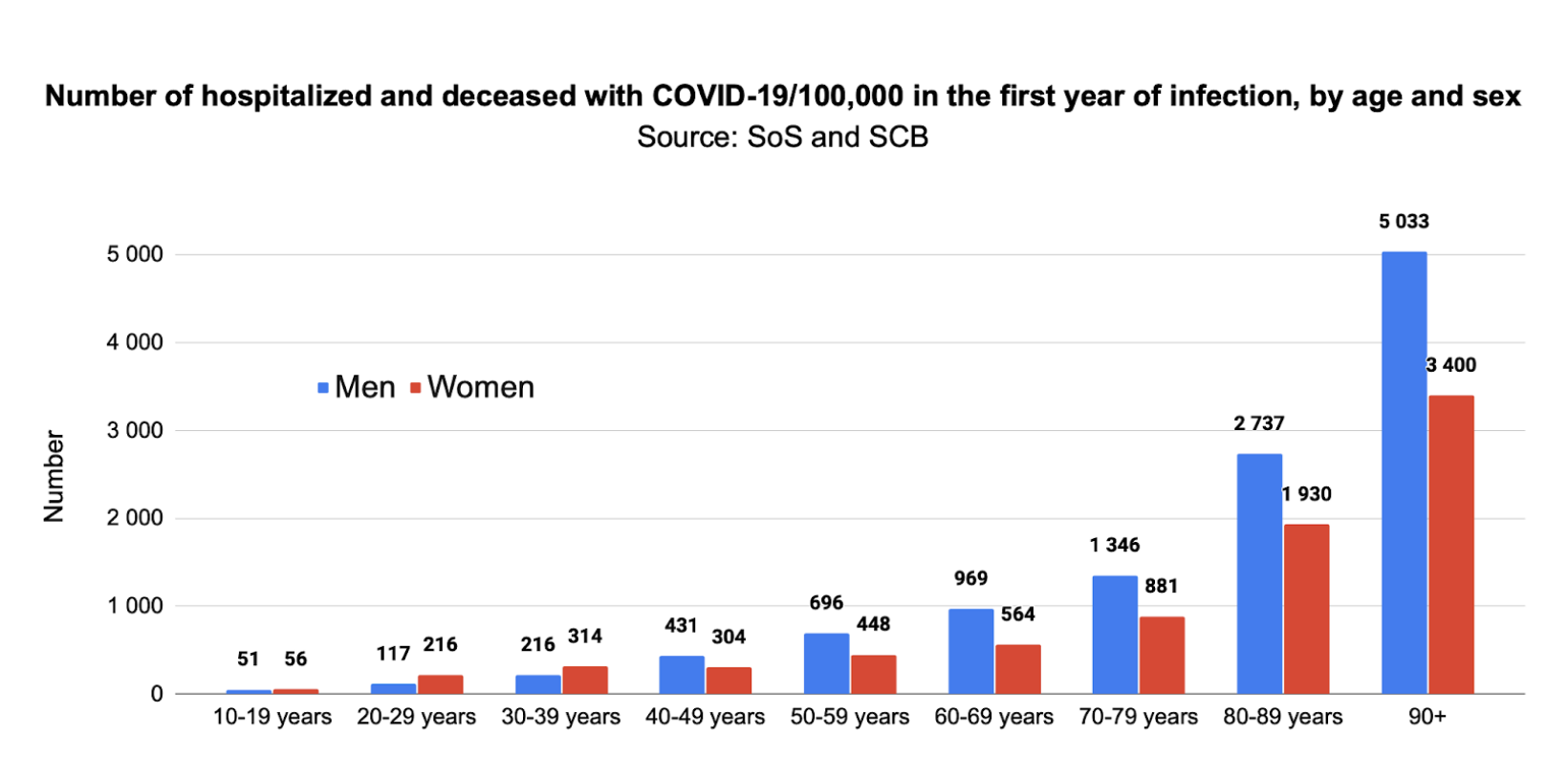
Our analysis was conducted in three steps. In step one, we estimated the number of people per 100,000 who were hospitalized or died with Covid-19 infection during the first year of the pandemic. We first used data for the number of people aged 10 years and older who were hospitalized with (n=81,864) or who had died from (n=12,111, total n=93,975) Covid-19 in hospital from March 2020, the first month of significant infection, to June 3, 2022, based on age and sex.2,3 In addition, by May 30, 2022, n=4,488 people had died from Covid-19 in another setting (total n=16,599),4 and we make the assumption that all were aged 10 years and older (a child aged 0-9 years is less likely to die outside hospital). Since the age of non-hospitalized cases was unknown, we assumed a similar age distribution to the hospitalized group, assigning cases to age groups accordingly.
To estimate hospitalizations and deaths with Covid-19 in the first year of infection, March 2020 – February 2021, we adjusted the total number of hospitalizations and deaths aged 10 years and older up to June 3, 2022 (n=98,463) by the proportion in the first year (53.2%, n=51,338) of all hospitalizations with Covid-19 for all ages up to June 3, 2022 (n=96,522),3 resulting in n=52 370 cases aged 10 years and older. Using population as of December 31, 2020,5 we could then estimate the number of hospitalized and deceased with Covid-19/100,000 over the first 12 months according to age and sex:
In step two, we estimated the number of persons who experienced at least one SSSE during the first year of vaccination/100,000 vaccinated according to LV’s definition of a serious adverse event:
A report is considered serious if the suspected side effect leads to death, is life-threatening, involves hospitalization or extended hospitalization, leads to disability, causes congenital malformation or other medically important event.6
The Swedish Covid-19 vaccination started on December 27, 2020. We included reports of SSSEs until December 23, 2021. During this period, LV deemed n=8,496 of reported adverse events to be SSSEs.7,8,9
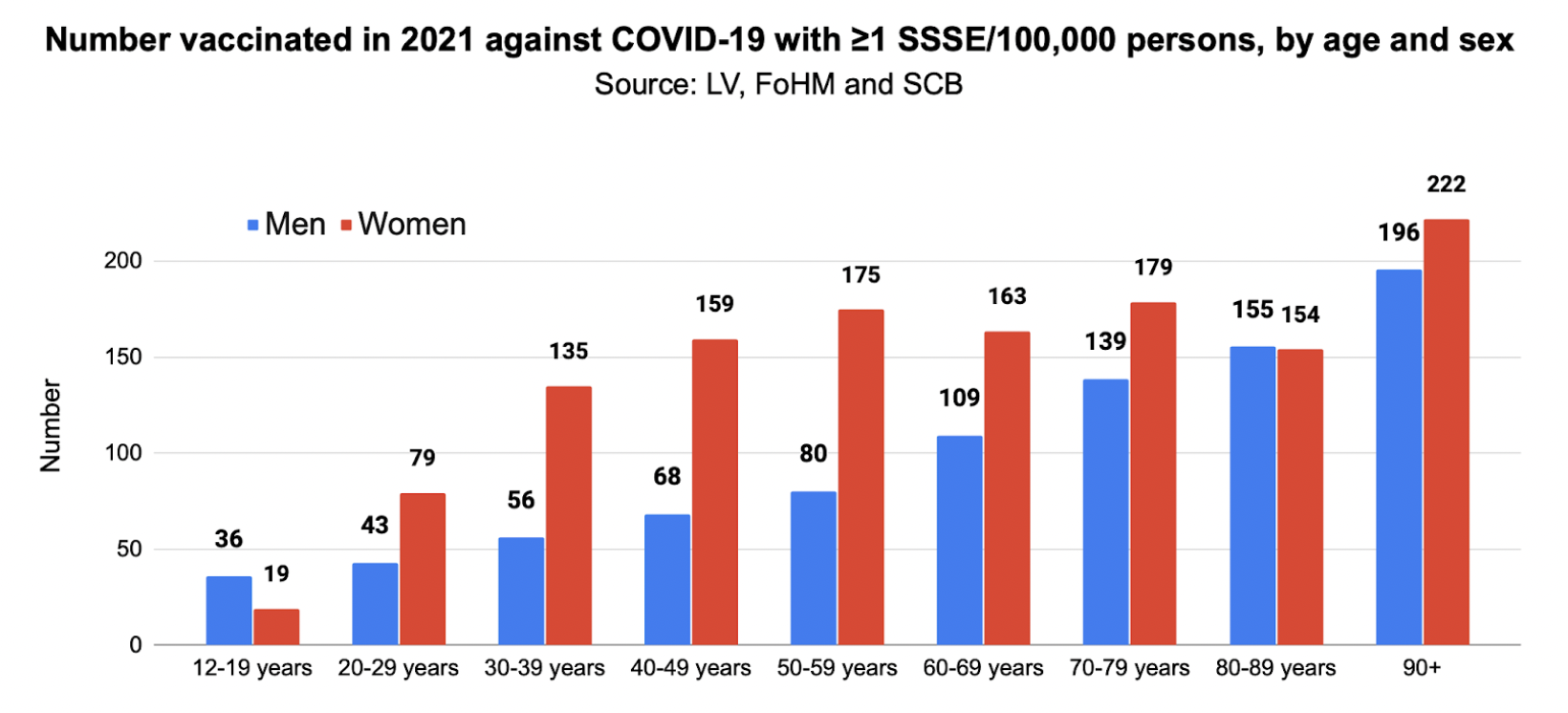
To estimate the number of cases of MAB per vaccinated person in the groups, we used data from FoHM.10 During the first year of vaccination, 85.4 per cent of the population aged 12 and over had received at least one dose of a vaccine. However, we lacked data on age distribution and sex, but were able to calculate the proportion vaccinated by age by combining the 85.4 per cent figure with data on the proportion vaccinated in different age groups up to June 19, 2022 and adjusting for the increase in vaccination coverage from December 23, 2021 to June 19, 2022.10 Based on the population at December 31, 2021 by age and sex,5 we were able to calculate the number vaccinated by age group and sex.
Since FoHM data showed that proportionally more women than men had been vaccinated, we adjusted for this in the final model – number increased for women and decreased for men so that proportion of vaccinated women is 1.0314 times that of men for all age groups. This resulted in the following:
Finally, in step three, we calculated the number of SSSEs per hospitalized or deceased with Covid-19. The reporting rate of SSSEs in Sweden might be as low as one to two per cent.11,12 Since the exact reporting rate for Covid-19 vaccine SSSEs is unknown, we tested outcomes using an assumed five per cent, 10 per cent or 25 per cent SSSE reporting rate. Thus we could estimate the number that experienced at least one SSSE/person hospitalized with Covid-19, according to age and sex:
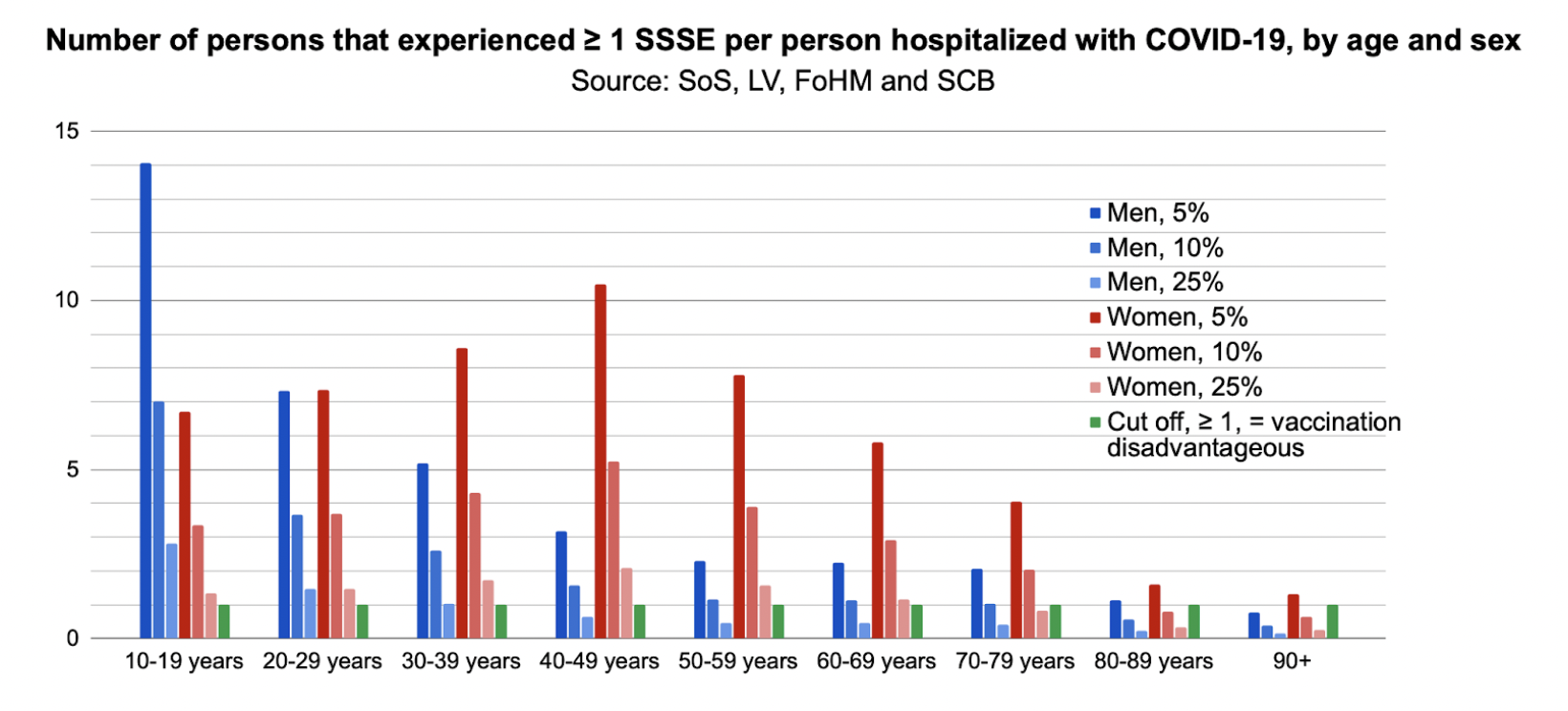
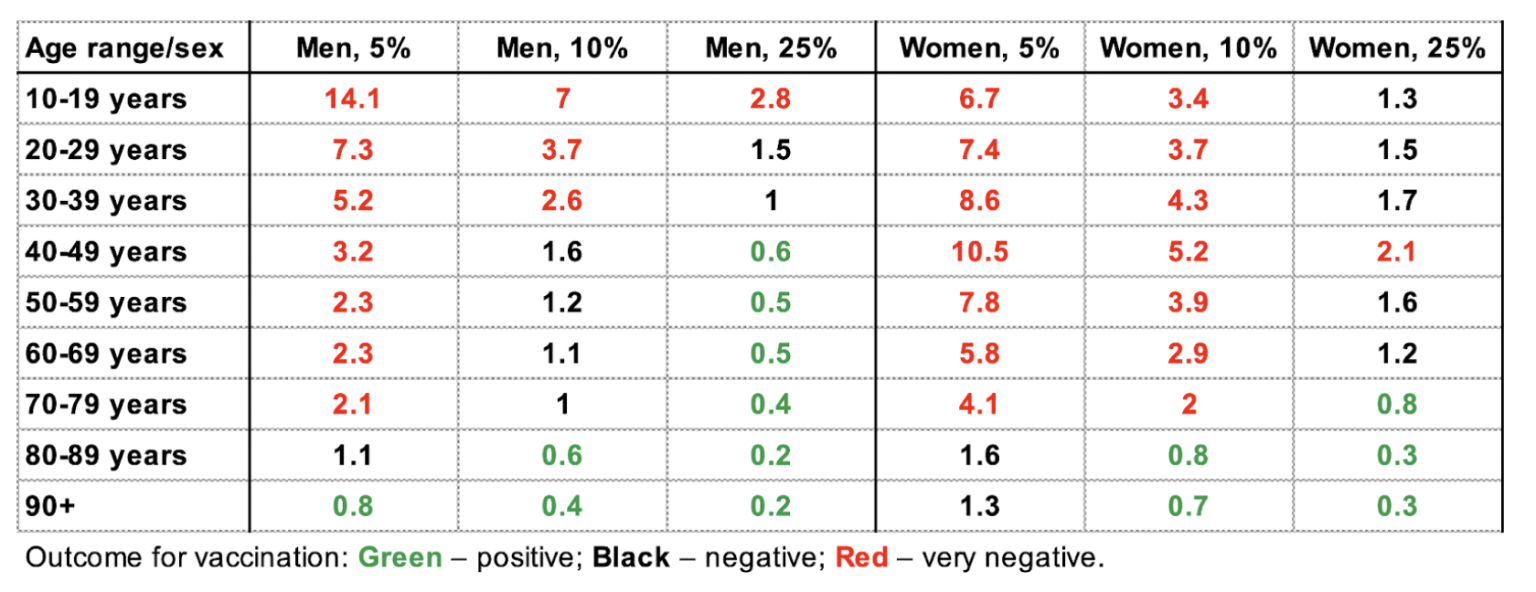
A value >1 suggests that Covid-19 vaccination was disadvantageous for that group. Women and men aged 10-79 years have been particularly affected by SSSEs. Assuming a five per cent SSSE reporting rate, men aged 10-19 years were the most disadvantaged, and experienced a 14.1 times higher incidence of SSSEs than hospitalization with Covid-19. Hence, risks of vaccination exceed the potential benefits in the majority of groups and vaccination only appeared to be beneficial in men aged over 90 years. Assuming a very conservative estimate of a 25 per cent SSSE reporting rate, risk outweighs benefit for men younger than 40 and women younger than 70.
Though we have made use of publicly available data, the full data set required to accurately determine the risk-benefit of Covid-19 vaccination is only available to the Swedish authorities. In the interests of public health, we implore the authorities to conduct a fully transparent and independent expert review of the available data to provide accurate morbidity, mortality and risk-benefit analyses.
- Sven Román, MD, Child and Adolescent Psychiatrist, since 2015 Consultant Psychiatrist working in Child and Adolescent Psychiatry throughout Sweden
- Anette Stahel, MSc in Biomedicine, former Cancer Researcher at the University of Skövde
- Jonathan Gilthorpe, PhD, Associate Professor in Cell Biology, Umeå University
- Johan Eddebo, PhD, Researcher in Digitalization and Human Rights, Philosophy of Religion, Uppsala University
- Niklas Lundström, PhD, MSc in Engineering Physics, Associate Professor in Mathematics, Umeå University
Conflict of interest statement: None declared.
References
- Fraiman, J, Erviti, J, Jones, M, Greenland, S, Whelan, P, Kaplan, R M & Doshi, P (2022) Serious Adverse Events of Special Interest Following mRNA Vaccination in Randomized Trials SSRN
- Socialstyrelsen (2022) Statistics on COVID-19 Official information from SoS
- Socialstyrelsen (2022) Statistik om covid-19; Statistik om avlidna i covid-19 Official information from SoS Available via Sven Román at romansven@gmail.com
- Socialstyrelsen (2022) Statistik om covid-19; Statistik om slutenvårdade patienter med covid-19 Official information from SoS Available via Sven Román at romansven@gmail.com
- Statistiska Centralbyrån (2022) Statistikdatabasen – Folkmängden efter Ålder och Kön År 1860 – 2021 Official information from SCB
- Läkemedelsverket (2021) Comirnaty – Svenska Handlagda Rapporter om Misstänkta Biverkningar – 2021-11-17
- Läkemedelsverket (2021) Comirnaty – Svenska Handlagda Rapporter om Misstänkta Biverkningar – 2021-12-23
- Läkemedelsverket (2021) Spikevax – Svenska Handlagda Rapporter om Misstänkta Biverkningar – 2021-12-23
- Läkemedelsverket (2021) Vaxzevria – Svenska Handlagda Rapporter om Misstänkta Biverkningar – 2021-12-23
- Folkhälsomyndigheten (2022) Statistik för vaccination mot Covid-19; Data som statistiken ovan bygger på kan laddas ner här (excel) Official information from FHM Available via Sven Román at romansven@gmail.com
- Jönsson, A K, Jacobsson, I, Andersson, M & Hägg, S (2006) Stor Underrapportering av Hjärnblödning som Läkemedelsbiverkning LT 103(45): 3456-3458
- Rydberg, D M, Holm, L, Engqvist, I, Fryckstedt, J, Lindh, J D et al (2016) Adverse Drug Reactions in a Tertiary Care Emergency Medicine Ward – Prevalence, Preventability and Reporting PLOS ONE 11(9): e0162948