Table of Contents
Dr. Guy David Hatchard
Guy is an international advocate of food safety and natural medicine. He received his undergraduate degree in Logic and Theoretical Physics from the University of Sussex and his Ph.D. in Psychology from Maharishi University of Management, Fairfield Iowa. He was formerly a senior manager at Genetic ID, a global food safety testing and certification laboratory. His published work uses the statistical methods of the physical sciences to analyse social data.
An influential cross-party group of UK MPs has raised concerns that the Medicines and Healthcare Products Regulatory Agency (MHRA) knew of serious cardiac side effects of COVID-19 vaccines, including myo/pericarditis and clotting, but failed to alert the public for several months between February and June 2021. During this time, over 24 million people received the COVID-19 vaccine in the UK alone, unaware of the risks.
The UK situation finds an exact parallel in New Zealand. An OIA request H202117570 published on January 12th 2022 confirms that Medsafe, our medicines regulatory authority, was made aware of an extensive range of side effects of the Pfizer COVID-19 vaccine prior to its provisional approval. This included the information contained in the damning Cumulative analysis of post-authorization adverse event reports of Pfizer bnt162b2 dated 28 February 2021. Yet Medsafe failed to advise the public of any risks for some months, instead telling the public COVID-19 vaccines were beyond doubt ‘safe and effective’.
As late as December 2021, Director General of Health Dr. Ashley Bloomfield, whilst then belatedly admitting the risk of myo/pericarditis, was, as we reported in May last year, minimising its possible prevalence in his public statements and communications with District Health Boards.
MPs Demand Investigation into MHRA’s Approach to Vaccine Safety
A revealing article in the UK Daily Telegraph entitled “Medicines regulator failed to flag Covid vaccine side effects and must be investigated, say MPs” reports that the All-Party Parliamentary Group (APPG) of 24 MPs on pandemic response and recovery has written to the Health Select Committee stating that “far from protecting patients” the regulator MHRA operates in a way that “puts them at serious risk.”
The group also warned that the MHRA Yellow Card reporting system – which encourages patients and doctors to flag-up medicine side effects – “grossly” underestimates complexities, and in some instances picks up just one in 180 cases of harm. In reply, Steve Brine, the health committee chairman, has said an inquiry into patient safety is “very likely”.
How is it possible that medicines regulators are failing in their duty and endangering public health?
APPG has submitted concerns that MHRA regulation of medicine is funded by the pharmaceutical industry and said the body had shifted from focusing on scrutiny to trying to help drugs get approved. Dame June Raine, the chief executive of the MHRA, who announced she would be stepping down last week, has previously said the agency was transitioning from “watchdog to the enabler,” a phrase which the APPG said warranted its own investigation.
Undermining Public Trust: The Conflict of Interest and Evasion in Drug Safety Regulation
The APPG said that concerns raised directly with the MHRA had been met with “an habitually dismissive and evasive response”.A phrase that aptly fits the tone and substance of Medsafe safety reports, which doggedly maintain to this day against all evidence that record number of reports of vaccine harm and death lodged with CARM (Centre for Adverse Reactions Monitoring) are unrelated to Covid vaccination. An indefensible position.
Medicine regulators, including Medsafe, are funded by the pharmaceutical industry they are supposed to regulate, which fosters a cosy if not corrupt relationship. Much is said about revolving doors between regulators and industry. Whilst this is true of countries with significant pharmaceutical manufacturing, the problem is more extensive than that. Global supply chains ensure that most countries end up mistakenly relying on lax or biased regulators based far away in other jurisdictions for a heads up on safety.
Often, the information is not originating from regulators at all. ICMRA (the international coalition of medicines regulatory authorities), of which Medsafe and MHRA are members, is largely funded by the global pharmaceutical industry. It operates a database providing information on drug safety and risks to its members. The database ensures that drug approval is semi-automated from the international level, which undermines the independent vigilance of national bodies. It provides an illusion of safety without the substance to guarantee freedom from risk to public health.
Rethinking Drug Safety: The Urgent Need for Transparency and Rigor in the Approval Process
The failure to publicly acknowledge the risks of COVID-19 vaccines shows what an unthinking process drug approval has become. The problem extends to a wide range of novel drugs. For example, the MHRA recently said it would investigate why very widely prescribed blood thinners were causing dangerous side effects in between two and five per cent of patients. In other words, as many as 5 out of 100 patients are being seriously affected by drugs MHRA had previously approved—an unprecedented stratospheric rate of risk. This augurs a catastrophic breakdown in drug safety regulation.
The introduction of mRNA COVID-19 vaccines raises even more serious safety concerns. The completely novel class of mRNA and other biotech vaccines which edit the command and control mechanisms of our immune system have been introduced despite early warning signals of serious harm at high rates. There was no long-term testing data because of the rapid response to the pandemic but crucially nor was there any adequate on-going plan in place to monitor the missing long-term effect data according to the canons of accepted risk assessment and criteria of causality.
Regulators like MHRA and Medsafe simply failed in their safety role and compounded this mistake in an apparent frenzy of ‘safe and effective’ public relations hype. How could they do that? They fell for the biotechnology myth promoted by PR gurus. In essence, the idea that biotechnology is so precise and accurate that any desirable trait can be enhanced scientifically and safely. A gobbledegook and totally at variance with published research.
Where does this leave New Zealand?
In 2023, we had the lowest birth rate ever in New Zealand, 26% below what is required to maintain our population. Our death rate is still running at 8% above the longer-term rate. Life expectancy has stalled. These are canary in the coal mine figures.
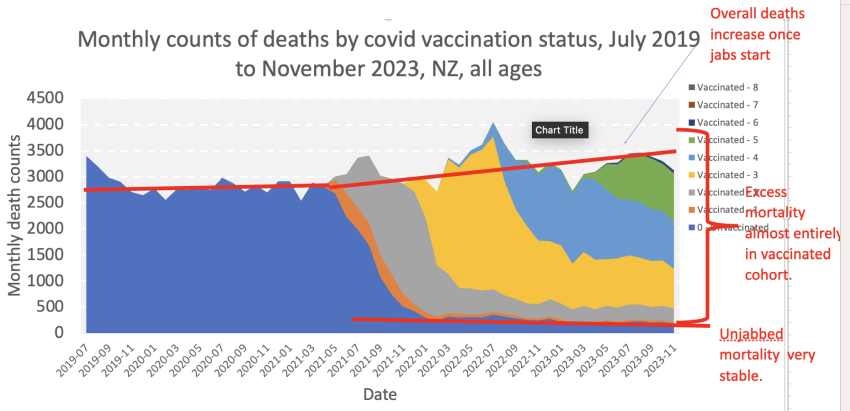
Official mortality data by vax status released on February 15 under an Official Information Request HNZ00033573 show the excess deaths are almost entirely among the vaccinated, while the death rate of the unvaccinated remains stable.
These figures should raise the alarm about the rapid introduction of biotechnology in healthcare. Yet Health New Zealand, the courts, and the government are still pursuing a campaign to discredit a sincere and principled whistleblower raising the alarm about excess deaths, whilst ignoring their own recently released figures, which, as the chart above indicates, verify and amplify the serious concerns highlighted by the whistleblower. Love is blind. The medical love affair with biotechnology needs to be put under the microscope. It is misguided. Health service administrators, drug regulators, and doctors alike need to face up to past mistakes and their own data illustrating the reality of declining public health.