Table of Contents
Marcus Richards
Jonathan M Schott
UCL
Marcus Richards is a Programme Leader at the Medical Research Council Unit for Lifelong Health & Ageing
Jonathan Schott is Professor of Neurology at the Dementia Research Centre, UCL Institute of Neurology, and Honorary Consultant Neurologist at Queen Square.
This article is part of the Insight’s Uncharted Brain series.
In March 1946, just months after the end of the second world war, James Douglas began a pioneering and extraordinary study. Based on a representative sample of 5,362 babies all born in the same week of that month, the study began as a one-off investigation of the cost of childbirth and the quality and efficiency of obstetric services. From there it became the longest continuously running study of health over the human life course in the world.
The Medical Research Council National Survey of Health and Development (NSHD), also known as the British 1946 birth cohort, continues to this day and the information enables us to glean new understandings about health – including the workings of the brain and the development of Alzheimer’s disease. We have been privileged to continue Douglas’s work and, indeed, today we still use the data he began gathering over seven decades ago.
In 2016, a sub-study of 502 people from the cohort, known as Insight 46, was started specifically to address brain ageing and dementia, and their life course influences. Using cutting-edge imaging and AI technology, we have been observing their brains ever since.
The results from our studies have revealed several important insights, including:
- Cognitive function in childhood relates to cognitive performance 70 years later.
- Education does not just increase opportunities but is significantly associated with brain health in later life.
- Midlife appears to be the time when hypertension and cardiovascular risk may influence dementia risk
- While weight gain in midlife has many adverse health implications, weight loss in later life may in some cases be a sign of impaired brain health.

This story is part of Conversation Insights
The Insights team generates long-form journalism and is working with academics from different backgrounds who have been engaged in projects to tackle societal and scientific challenges.
How it all began

But when the study began, its remit was quite specific. The UK government, anxious to rebuild infrastructure after the second world war, set out to discover what might be putting people off having children. The national birth rate had been steadily declining since the mid-19th century and had indeed been of concern since at least the 1920s. Yet the need to grow the postwar labour force made this a particularly urgent goal. This was also linked to concerns about infant mortality, the availability and quality of relevant support for families, and how far this promoted the health and survival of mothers and babies.
To investigate these issues, the Population Investigation Committee and Royal College of Obstetricians and Gynaecologists appointed Douglas – a physician with an interest in public health, noted for his studies of WWII air raid effects on the mental health of children. Douglas recruited health visitors who set out to interview every mother in mainland Britain who gave birth during one week of March 1946 – a major achievement in any circumstance let alone in a country that had just been battered by war and was still living with food, fuel and clothing rationing.

The health visitors found 13,687 mothers who were asked about their experience of medical and social care and its costs (this was two years before the NHS), health and survival of the baby, and the circumstances of the family. The results were written up as a book called Maternity in Great Britain.
The results highlighted the benefit of health visits and community infant care services, how infants of poorer families suffered worse health and shorter survival, with the cost of childbirth eating disproportionately into their incomes. This was years before the availability of computers, so the information was processed by hand, again an extraordinary achievement.
In her book, The Life Project, science journalist Helen Pearson quotes some of the questions asked in the survey. The questions reveal a lot about society at that time.
- Who looked after your husband while you were in bed with this baby?
- Were you able to get your full extra ration of a pint of milk a day?
- How much did you spend on vests, napkins, petticoats, bootees, bonnets, shawls, knickers and rubber sheets for baby?
- And how much did you spend on smocks, corsets, nightdresses, knickers and brassieres for yourself?

Although the postwar baby boom eased the birth rate crisis, Douglas wanted to see if the health inequalities he had found persisted. To bring the study to a more manageable size, a sample of 5,362 was selected for follow up.
The UK is exceptionally rich in these birth cohorts. They encompass the best part of a century and are the envy of the world. In descending order of age these are, after NSHD, the National Child Development Study born in 1958; the 1970 British Cohort Study; Next Steps, born in 1989-90; the Millennium Cohort Study; and the Children of the 2020s Study. These cohorts will allow us to see which outcomes are constant over that time. They can also be combined if very large numbers are needed, for example, for rare outcomes or certain kinds of genetic studies
Interestingly, there seems to be a stronger relationship between education and everyday literacy and numeracy in the 1958 cohort, than in the 1946 cohort. Why? Probably because in the intervening 12 years the government raised the minimum school leaving age from 15 to 16 years. Following this, there was a substantial increase in the proportion of children leaving school with at least some qualifications.
Douglas had arranged for study members to be cognitively tested at school and showed that children from middle-class families were significantly more likely to go to grammar schools than children of working-class families who scored at least as high on the tests. This reflected an ongoing policy concern known as the “waste of talent” crisis, as highlighted in the 1959 Crowther report.
Impossible to estimate retrospectively, these measures of cognitive function were to prove invaluable, not only for studies of their early determinants (such as health, growth and family circumstances) but also as major predictors of mental ageing.
The history of the 1946 cohort has been punctuated by technological changes, including in blood testing, imaging and genetics. Recent advances in wearable and remote technologies, such as watches that track activity, heart rate and sleep patterns, promise a new era of data on health, cognitive functioning and activity to be collected in incredible detail and in the home setting, which will be ever more important as the cohort ages.
Every year we send participants a birthday card with a newsletter that summarises key findings from the cohort over the past year. It’s a fun team ritual to sit around a long table chatting while stuffing the envelopes. The design of the card reflects changing artistic tastes over these years.

Tracking subtle variations
When members of the cohort reached their mid-30s, Mike Wadsworth, the then director of the NSHD, focused the study on physical and mental health, particularly the measures that show subtle variation in the general population, such as blood pressure, lung function, physical performance and emotional symptoms. This approach was enhanced by the subsequent directors Diana Kuh and Nish Chaturvedi.
Cognitive function was picked up again at age 43 with a new emphasis on skills vulnerable to decline, such as memory, concentration and mental speed. These measures, along with those of physical and psychiatric health and their life course influences, form the platform on which we built our brain study, Insight 46.
We are both used to interacting with research participants, so never forget that there are human beings behind the numbers. We feel humbled at how much the participants are prepared to give: the questions, tests, medical devices and investigations, blood draws – and now brain scanning and, in a proportion, lumbar punctures.
We in turn have the responsibility of knowing so much about these people, of protecting their anonymity and providing a duty of care when we detect problems. This gives us the feeling of being “future detectives”, looking forward, yet guided by a road map only made possible by 76 years of commitment from study members.

This article is accompanied by a podcast series called Uncharted Brain: Decoding Dementia which examines new research unlocking clues to the ongoing mystery of how dementia works in the brain. Listen to the trailer via The Anthill podcast ahead of the series launch on November 16.
What is dementia?
Dementia is an ancient word meaning “out of mind”, but today it refers to a syndrome of acquired (not present from birth), progressive cognitive impairment, severe enough to interfere with everyday activities such as planning meals, managing bills and medicines, and housekeeping.
As cognitive impairment worsens, these activities become more impaired, eventually disrupting basic self-care such as dressing and bathing. Sometimes this is accompanied by depression, paranoia, aggression, wandering or reversed sleep-wake cycles. Dementia is therefore very different from the mild cognitive changes that occur in normal ageing.
Alzheimer’s disease is the commonest form of dementia. On a biological level, a key process is the depositing of beta-amyloid protein in the brain. This is a protein that comes from the fatty membrane surrounding nerve cells. It is chemically sticky, gradually clumping together and interfering with nerve function and triggering inflammation. These clumps gradually gather between the nerve cells in the brains of people with Alzheimer’s disease and become plaques – hard, insoluble accumulations of beta-amyloid proteins.
These plaques are thought to be an early hallmark microscopic feature of Alzheimer’s. However amyloid plaques in themselves are not sufficient to cause dementia which is more closely related to accumulation of another, likely downstream, protein called tau which clumps within nerve cells in the form of tangles.
Accumulation of these proteins leads to nerve cell death which leads to brain shrinkage (atrophy) which can be seen using MRI scans. The diagnosis of Alzheimer’s dementia remains predominantly clinical, requiring evidence of decline over time in at least two cognitive areas, such as memory, language, attention or problem solving. Contemporary criteria also involve investigations including MRI or CT brain scanning and, in some cases, spinal fluid or positron emission tomography (PET) scans. PET scans can be used both to visualise and quantify abnormal protein deposition within the living brain. For Insight 46, we use a tracer that is injected into the body, enters the brain and which highlights where any amyloid is accumulating.
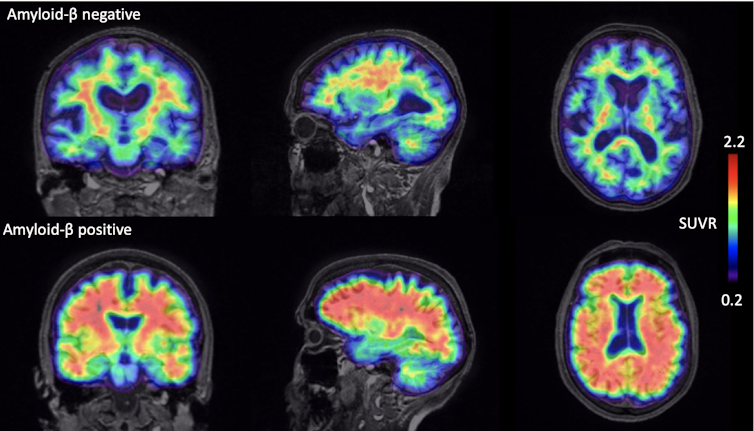
Alzheimer’s disease is only one of many forms of dementia. Other causes include other neurodegenerative disorders due to the accumulation of different proteins, and cerebrovascular disease where the blood supply to the brain is interrupted, for example, from blood vessel narrowing, blockage or bleeding.
The beginning of Insight 46
While developing what was to become the mental ageing research programme for our team, we acknowledged that it would be an exciting new direction to scan the brains of NSHD participants.
Previous work at the Dementia Research Centre at the University College London Institute of Neurology had shown that MRI scans from patients with rare genetic forms of Alzheimer’s disease showed excess brain shrinkage occurred before symptoms started.
In addition, UCL had installed the UK’s first scanner that allowed MRI and PET to be measured simultaneously. As NSHD participants were approaching the age of 70 and still relatively healthy, this raised an exciting possibility: our two teams could combine forces and combine the uniquely rich life course data with state-of-the-art scanning to explore the brain changes that occur before symptoms become apparent in a birth cohort – something that had never been done before.
But along with our excitement came apprehension: study members had attended research clinics, to have their hearts, blood vessels and bones scanned. Would they now come to London to be injected with a radioactive tracer, then lie in a scanner for an hour? To find out we hired a focus group expert to run sessions with participants. This is some of their feedback:
You tested our hearts, bones, why not our brains?
I think anything that we can do to try and limit, reduce the prevalence of Alzheimer’s, dementia, absolutely has my 110% support.
I’m happy just even talking about it now, I feel less scared …
Now that we felt more confident that this could work, we needed to decide who to invite. At that time around 3,000 participants were spread over mainland Britain. We worked out that a sample of 500 people would be ideal to study subtle changes in the brain and their influences, and that this was feasible. If we could see two people a day for three days per week then it would be possible to see 250 people per year, and so to see everyone twice within a five-year timeframe.
We guessed that those who previously came to clinic would be more willing to travel to London, stay overnight, then spend a day at our clinical research lab at UCL. We were careful to make sure there were no reasons someone shouldn’t be scanned, such as claustrophobia or metal implants. Then the invitations went out, with arrangements for travel and hotel, details of what to expect – and even a brochure for local attractions and amenities.
Those who agreed to attend had a range of different assessments including a clinical interview, a blood draw, an hour of cognitive testing, a physical and neurological examination and tests of hearing, sight and smell. Finally, came the visit to the scanner.
Once the blood test results are processed and the MRI scan reviewed by an expert, if any clinically relevant problems are identified these are fed back to the participant and their general practitioner, with advice given as needed.

As the study progressed, and following feedback and close liaison with participants, we have added more tests including detailed assessment of heart function, offering people a lumbar puncture (which about 30% have agreed to) and monitoring activity and sleep using a smartwatch.
The journalist David Ward is an NSHD participant. We are fiercely protective of participants’ anonymity, but David has always been willing to identify himself and has written movingly about what it is like to be studied for 76 years. “It followed me through school, testing my brain, checking on my development and keeping tabs on my educational progress. It stayed with me through university, noting that I nearly died of a pneumothorax just before finals in 1967, and then recorded that I had married, become a dad and wandered around the country to new jobs.”
I am happy to boast that I have been described as one of the best-studied people on the planet. And I’m quietly proud that information about me, ranging from how many pairs of bootees I had at birth to the state of my memory now, has appeared in at least eight books and 700 other publications.
Twice my failing brain has been tested by a rigorous battery of cognitive tests lasting the best part of a day. But as I struggled to crank up my memory and to persuade both sides of my brain to collaborate, I thought of the people I meet at regular sessions of Singing for the Brain run by the Alzheimer’s Society. It might just be that all those tests and the data they provided will eventually make a tiny contribution to halting the progress of a condition that frightens us all.
What have we found so far?
We cannot attempt to cover all our numerous research findings to date, as there have been so many. But our investigations have highlighted some key themes.
1. Amyloid accumulation starts before symptoms
We found that around 18% of “cognitively normal” people from the cohort had amyloid PET scans like those seen in people with Alzheimer’s disease – a finding that tallies with other studies in people around the world who don’t have symptoms. These individuals also had slightly lower performance on sensitive tests of cognition and slightly increased rates of brain shrinkage.
While the significance of the finding for amyloid frequency is unclear – and hence our protocols and consent processes mean that unlike some MRI findings we do not give the results to participants – we think that these individuals are at higher risk of developing cognitive impairment in the future, something we plan to look out for closely in the years to come.
2. Child cognitive tests indicate brain function later in life
We found that cognition assessed in childhood predicted cognition around 60 years later. This is consistent with earlier findings for the whole cohort, suggesting that some aspects of cognitive performance are stable over a lifetime. This matters because cognitive function is not just about the mind – it helps to shape everyday skills, supports quality of life and ultimately predicts how long we live.
However, the level of cognitive performance can be potentially improved. In the same report, education and occupation in midlife predicted later cognition after taking account of childhood cognitive scores. We had seen this in the whole cohort too, which counters an old argument, still sometimes made, that education is nothing more than a marker of IQ. In other words, level of education and type of occupation can positively affect cognitive performance in later life regardless of cognitive skills in early childhood.
It also emphasises that education does not just increase opportunities but has a significant effect on brain health.
3. Importance of early heart health checks
Some of the first publications from Insight 46 showed that high and rising blood pressure in those aged in their 40s and – in some cases their 30s – predicted smaller brain volume. There are several possible mechanisms for this, including microstructural damage from high blood pressure and a higher burden of small blood vessel damage in the brain. The latter is thought to be a marker of brain frailty, raising the risk of stroke, dementia, depression, impaired mobility and death.
Similar outcomes were seen in relation to heart health in general, using an index that includes blood pressure, use of anti-hypertension medication, diabetes, smoking and high body weight. Conversely, falling blood pressure in later life may in some cases be a marker of poor brain health.
Similar findings may also apply to body weight. A follow-up analysis found that declining body weight in the two years before the scan predicted amyloid.

These findings have significant implications for public health, suggesting that routine checking of heart health, and blood pressure, in particular, may need to start much younger than is typically recommended – probably at or before the age of 40.
4. A blood test for Alzheimer’s disease
Most experts will agree that when we have new drugs for Alzheimer’s disease, they are likely to have maximum benefits if taken early in the disease, and preventing the onset of cognitive decline would clearly be preferable to trying to slow or halt memory decline that has already started. It is unlikely that the expensive PET scans we are conducting in Insight 46 will be able to screen whole populations, so there is much interest in developing blood tests instead.
Using state-of-the-art methods sensitive enough to detect 1g of salt dissolved in one million trillion litres of water, we were able to show that a blood marker is capable of detecting amyloid in the brain with about 85% accuracy. We are currently looking at a range of new blood tests that seem to be even better at detecting amyloid, and at even lower cost.
The prospect of new drugs that can clear amyloid from the brain provide even more reason to intensify efforts to identify amyloid pathology early, cheaply and at scale.
Studies using the whole NSHD cohort have also shown complex relationships between cognitive function and several bodily functions, including those of the lungs, bones and kidneys. This probably reflects biology shared between the brain and these organs. We are currently looking to see how these findings relate to the brain health measures we have made in Insight 46. A similar “common cause” story applies to depression and cognitive function, and we are currently looking into how depression relates to the brain.
On the other hand, health-related behaviour such as smoking, physical exercise and healthy diet genuinely seem to predict cognitive function (negatively for smoking, positively for exercise and diet).
We have been emphasising prediction of health problems, but it’s equally important to understand resilience. Why can some people navigate through or escape these problems altogether even though they are apparently at risk, from genes or certain disadvantages in life? Does it come down to pure luck? But luck is, of course, just another way of saying we don’t know something.
Uncovering what predicts Alzheimer’s
That brings us to retirement. Retirement is one of the most vivid life transitions we can experience. Yet we know surprisingly little about its effects on ageing, including mental ageing. Work provides many of the everyday things that sustain our brain health: physical activity, mental stimulation, the security of an income, a role in society and a structure to our daily lives. We risk losing these when we retire unless we can find ways to maintain or replace them.
With its life course design, NSHD provides an ideal opportunity for us to investigate the effects of retirement across a range of health outcomes, including brain health, and we hope to be able to report on this in the near future.
But perhaps the most profound contribution that Insight 46 can make is to uncover what predicts Alzheimer’s disease – one of the biggest causes of disability and dependency in older people. At the age of 76, the cohort study is still relatively young when it comes to examining this aspect. However, as participants continue to age, Alzheimer’s will inevitably become more common.
Indeed, some studies suggest that we have over a 30% chance of developing this condition if we live beyond our mid-80s. To reduce these odds, we need to be able to look back over the whole life course to see where we can best intervene. We mentioned education and leisure activities, heart health, and maintaining quality of life after retirement. But the richness of information provided by NSHD will open possibilities not yet even thought of.
So, to achieve these goals we need to keep NSHD and Insight 46 going. We have already started another wave of assessments that will increase the number of study members with dedicated brain investigations to 1,000. We want these to become the world’s first continuously followed cradle-to-grave studies of general health and brain health. Our aim is to keep building a whole-of-life model that others can complete after we ourselves have retired.
This will be a road map to guide hypotheses for future research but also enable travel in exciting new and unknown directions.
But ultimately the only way of knowing what is going on in the brain is to examine it after death – and we are humbled that already over a third of Insight 46 have signed up for postmortem brain donation.
The life course of NSHD will eventually be complete, as it will for all of us. However, that certainly won’t be the end, thanks to a rich body of data and evidence which will continue to flow from the UK’s different birth cohorts.
While we continue to plan future assessments, at the same time we look back over the incredible information that the 1946 participants have already provided. In doing so we can only imagine what James Douglas would be thinking if he could see where his study of the cost of childbirth is now – 76 years later and counting.

For you: more from our Insights series:
- Climate scientists: concept of net zero is a dangerous trap
- Sexual exploitation by UN peacekeepers in DRC: fatherless children speak for first time about the pain of being abandoned
- The public cost of private schools: rising fees and luxury facilities raise questions about charitable status
Marcus Richards, Professor of Psychology in Epidemiology, UCL and Jonathan M Schott, Professor of Neurology, UCL
This article is republished from The Conversation under a Creative Commons license. Read the original article.